
Newsroom
Hydrazine (N2H4) is a highly toxic organic amine that can spontaneously ignite or explode when exposed to strong oxidants, air, or high temperatures. It is classified as a Class B2 hazardous substance by the U.S. Environmental Protection Agency, the World Health Organization, and the International Agency for Research on Cancer, which underscores the significant risks it poses, highlighting the need for effective detection methods.
Fluorescence turn-on probes that utilize the excited-state intramolecular proton transfer (ESIPT) mechanism provide high sensitivity and reduce background interference, making them highly useful for detecting hazardous substances. However, current designs struggle with specificity for similar target analytes, and there is a lack of research on how modifying the substituents of the probes affects their sensing performance. Therefore, enhancing ESIPT-based probes by adjusting the properties of substituents remains a critical challenge.
To tackle this issue, a research team led by Prof. DOU Xincun from the Xinjiang Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences, has developed a design strategy for a zero-background fluorescence probe aimed at improving the ultrasensitive and specific detection of hydrazine. Their findings, published in Analytical Chemistry, focus on tuning the electron-accepting ability of the para-substituent within the recognition site, as well as the relative positioning of the proton donor and recognition site, to improve the probe's reactivity towards N2H4 and enhance the triggering of the ESIPT process.
In this study, the researchers designed a series of zero-background fluorescent probes (including m-Br-OH-BDMN, m-CH3-OH-BDMN, OH-BDMN, Br-BDMN, and p-Br-OH-BDMN) with dicyanoethylene serving as the recognition site. They regulated the electron-accepting ability of the para-substituent (-Br > -H > -CH3) on the dicyanoethylene and the positioning (para or ortho) of the proton donor (-OH) relative to the dicyanoethylene.
The researchers found that a stronger electron-accepting capability significantly enhances the reactivity of the recognition site. The probe only reacted with N2H4 to produce a hydrazone when the hydroxyl group was positioned ortho to the recognition site, which then triggered the ESIPT process, resulting in blue-green fluorescence emission. The probe m-Br-OH-BDMN, which has bromine as the electron-accepting group, demonstrated excellent detection performance for N2H4, featuring a low limit of detection (LOD) of 0.46 nM (14.72 ng/L), a rapid response time of 1 second, and superior selectivity even in the presence of 18 other interfering substances, including structural analogues of primary amines.
Additionally, the researchers proposed a silicon-based porous sensing material design strategy to efficiently adsorb and concentrate target substances, thereby improving molecular collision efficiency. This strategy effectively distinguishes N2H4 from ethylenediamine solutions and vapors within five seconds, without interference from other volatile vapors. This non-fluorescent probe design strategy provides new insights for the rational design of functional probes and high-performance sensing methodologies.
This study was supported by the National Natural Science Foundation of China, the Youth Innovation Promotion Association of the Chinese Academy of Sciences, and the Tianshan Talents Plan.
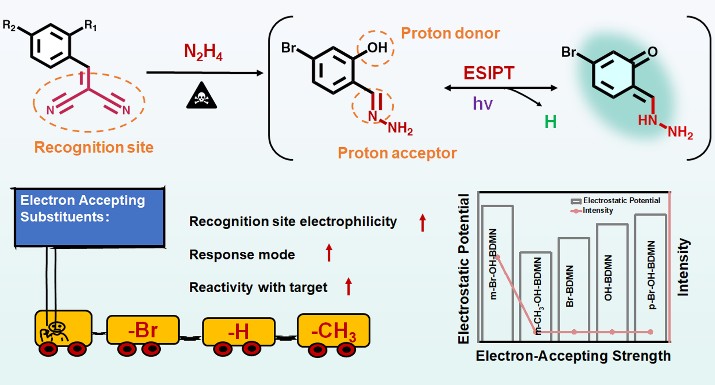
Schematic illustration of the designed strategy of the BDMN-based probe and the modulation of the electron-accepting strength of the para-substituent of the recognition site. (Image by the research group)
