
Newsroom
Photocatalytic water splitting is a clean energy technology that uses sunlight to split water into oxygen and hydrogen in order to produce green hydrogen—a clean fuel—without relying on fossil fuels. The process is driven by a photocatalyst.
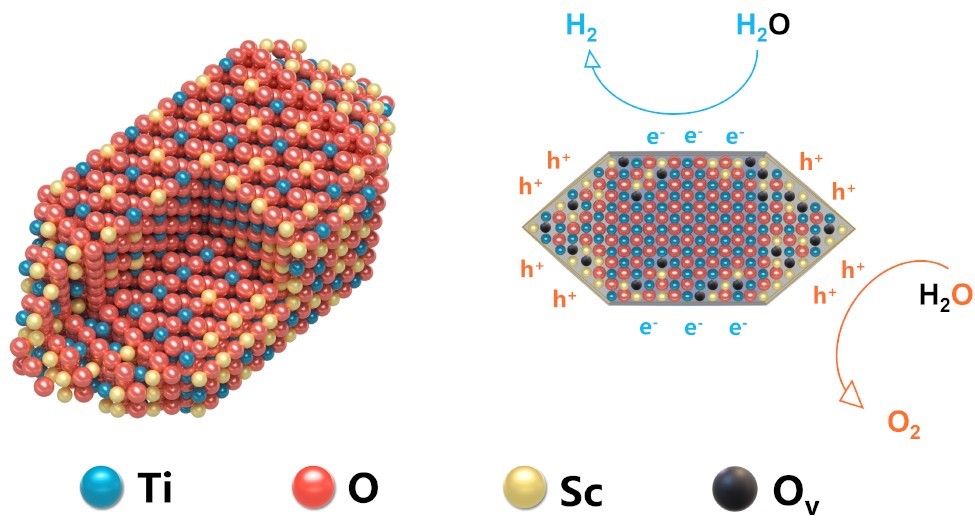
While titanium dioxide (TiO2) has long been studied as a promising semiconductor for photocatalytic water splitting, its efficiency has been hindered by rapid charge recombination and insufficient charge separation.
Now, however, a research team led by Prof. LIU Gang from the Institute of Metal Research (IMR) of the Chinese Academy of Sciences (CAS) has achieved a breakthrough in photocatalytic water splitting by developing a scandium (Sc)-doped titanium dioxide (TiO2) semiconductor in the rutile crystal phase.
The novel material demonstrated an apparent quantum yield (AQY) of 30.3%, which measures the percent of photons that lead to useful water splitting, and a solar-to-hydrogen (STH) efficiency of 0.34%, which indicates the percent of solar energy converted into hydrogen energy. Both values set new benchmarks for TiO2-based photocatalytic overall water splitting under ambient (non-pressurized, non-heated) conditions.
To overcome the challenges associated with TiO2, the research team employed a dual-strategy approach. First, Sc3+ doping effectively eliminated detrimental Ti3+ defects, which are known for trapping charges and causing energy loss. The team then engineered a facet junction between the (101) and (110) crystal planes, generating a built-in electric field that drives electrons and holes to separate facets—facilitating water reduction and oxidation reactions.
"This dual approach not only minimizes defect-induced charge recombination but also mimics the efficient charge separation mechanism of p-n junctions in photovoltaic cells," said Prof. LIU.
The findings underscore the significant commercial potential of Sc-doped TiO2, particularly given China's abundant titanium and scandium resources. With an established industrial supply chain for titanium dioxide and advanced rare earth processing capabilities, this innovation could pave the way for scalable and cost-effective hydrogen production.
"Our design strategy—suppressing defects and leveraging crystal anisotropy—aligns perfectly with China's resource strengths and industrial infrastructure," said Prof. LIU. The team now aims to enhance light absorption and integrate the material into scalable solar-driven systems.
This work was published in the Journal of the American Chemical Society and was financially supported by the National Natural Science Foundation of China, the National Key R&D Program of China, the Science and Technology Major Project of Liaoning Province, and the CAS Projects for Young Scientists in Basic Research.
Schematic diagram of TiO2 facet control and defect elimination (Image by IMR)
