
Newsroom
Tryptamine psychoactive substances, such as α-methyltryptamine (AMT), are monoamine alkaloids characterized by an indole ring structure. Rapid, highly sensitive, and specific identification of trace amounts of AMT is crucial for maintaining social stability, and ensuring public safety. However, accurately detecting AMT using specific fluorescent methods is challenging due to the presence of similar amine groups and benzene rings in various other amines.
To address this challenge, a research team led by Prof. DOU Xincun from the Xinjiang Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences (CAS) has developed a novel molecular probe strategy to enhance detection sensitivity and selectivity for AMT. Their findings, published in Analytical Chemistry, emphasize tuning the electron-withdrawing strength of the π-conjugate bridge to improve the reactivity of Schiff base-based fluorescence probes with amines.
In this study, researchers developed three Tricyanofuran (TCF)-based probes with different π-conjugated bridges—benzene, benzothiadiazole, and 2,5-dibromobenzene—tailored for amine-containing analytes. Among these, the aldehyde group in the probe with −C6H2Br2 as the π-conjugate bridge, denoted as BrFS-TCF, showed the highest electrostatic potential, making it the most effective for AMT detection due to its superior reactivity.
The optimized probe demonstrated remarkable performance, achieving a fluorescent detection limit of 13 nM, a colorimetric detection limit of 132 nM, and a response time of less than 0.1 seconds. Additionally, the integration of a convolutional neural network algorithm enabled the probe to distinguish AMT from other primary amines, further enhancing its specificity.
Moreover, the probe's reliability was validated through the detection of trace AMT in artificial saliva and solid residues, showcasing its potential for real-world applications. This innovative probe design and regulation strategy not only provides a new approach for the specific identification and discrimination of primary amine-containing drugs but also advances the development of methodologies for detecting trace hazards and illicit substances.
This study was supported by the National Natural Science Foundation of China, the Youth Innovation Promotion Association of CAS, the Tianshan Talents Plan, and the Key Research and Development Program of Xinjiang.
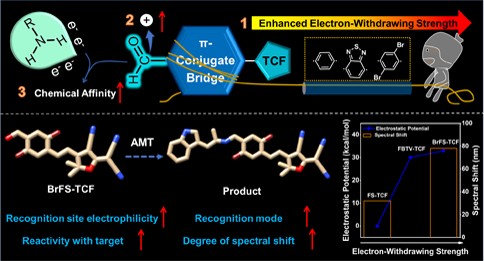
Figure: Schematic illustration of the designed strategy of the TCF-based Schiff base probe and the modulation of the electron-withdrawing strength of π-conjugate bridge. (Image by the research team)
