
Researchers from the Research Center for Eco-Environmental Sciences (RCEES) of the Chinese Academy of Sciences have developed a sustainable technology to selectively reduce nitrate to ammonium. This innovation delivers three benefits: It increases rice yield, reduces fertilizer usage, and mitigates nitrate pollution in groundwater. The findings were recently published in Proceedings of the National Academy of Sciences (PNAS).
Ammonia is a vital chemical used in fertilizer production and a potential high-density energy carrier. Historically, its production has heavily relied on the energy-intensive Haber process (N2+H2→NH3), which generates over 420 million tons of CO2 annually, equivalent to 1–2% of global energy emissions. Meanwhile, excessive fertilizer use and industrial wastewater discharge have caused severe nitrate (NO3-) pollution, posing significant risks to human health and ecological stability.
In recent years, ammonia production through electrochemical nitrate reduction has emerged as a green alternative. However, most current research is conducted under extreme pH conditions (either strongly acidic or strongly alkaline), while few studies explore neutral conditions. This limitation arises from the weak interfacial affinity of nitrate at neutral pH, leading to low reduction efficiency.
Drawing inspiration from the widespread presence of Fe (II) ions in nature, researchers at RCEES developed an in-situ regulation strategy for solid-liquid interfaces (Figure 1). FeOOH, a common hydroxylated iron oxide, serves as the Fe source, generating an in-situ Fe (II) ion layer upon electrical stimulation. This process limits electrostatic repulsion and enhances nitrate ion aggregation at the interface, significantly improving nitrate reduction efficiency.
Rice is one of the world’s three staple crops—along with wheat and maize—and feeds more than half the global population. It relies on ammonium as its primary nitrogen source. In rice paddies, irrigation water often contains abundant nitrate. To enhance nitrogen absorption and reduce reliance on chemical fertilizers, the researchers proposed a strategy of converting nitrate into ammonium (Figure 2). This conversion is particularly critical during the tillering stage, when rice absorbs over 90% of its nitrogen requirements. Laboratory pot experiments showed that this approach increased rice yields by more than 20% while reducing fertilizer use by 50%, representing a significant improvement over conventional practice.
However, nitrate naturally tends to reduce to nitrogen gas rather than ammonium, thus presenting a major challenge. To address this problem, the researchers relied on previous findings about FeOOH to introduce a single-atom iron catalyst into their electrochemical system. This catalyst demonstrated exceptional reduction capability, achieving over 90% selectivity for converting nitrate to ammonium. Furthermore, the researchers used advanced 15N isotope tracing technology to confirm that more than 80% of the nitrogen from the environmental nitrate was absorbed by the rice, ensuring a sustainable nitrogen supply for cultivation.
Since rice’s nitrogen utilization efficiency from chemical fertilizers is only 30–40%, much of the nitrogen in rice fields leaches into groundwater as nitrate, posing significant risks to drinking water safety. However, the selective reduction of ambient nitrate to ammonium developed by RCEES offers an innovative solution to this problem. By converting “harmful” nitrate into “beneficial” ammonium, this method prevents over 70% of nitrate from leaching into groundwater while enhancing nitrogen absorption by rice and reducing the need for external nitrogen inputs.
Cost-benefit analyses show that this approach reduces costs by 19% and increases revenue by 27% compared to traditional urea fertilization. These findings highlight the technology’s ability to simultaneously enhance food security while promoting environmental sustainability.
Led by researchers ZHU Guibing and LIU Chunlei from RCEES, this study marks the first use of electrochemical technology for treating agricultural irrigation water, paving the way for innovative solutions that improve agricultural productivity while preserving natural resources.
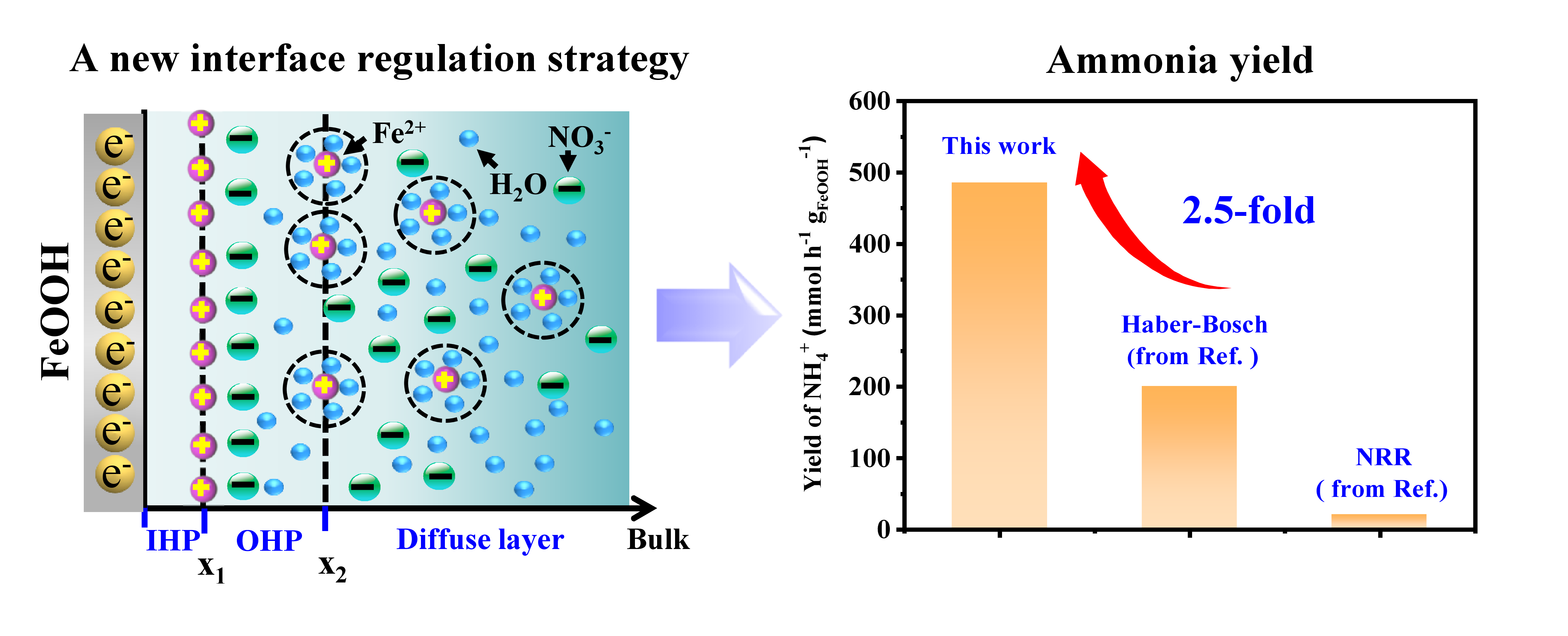
Figure 1 A new interface regulation strategy for high ammonia yield (Image by LIU Chunlei)
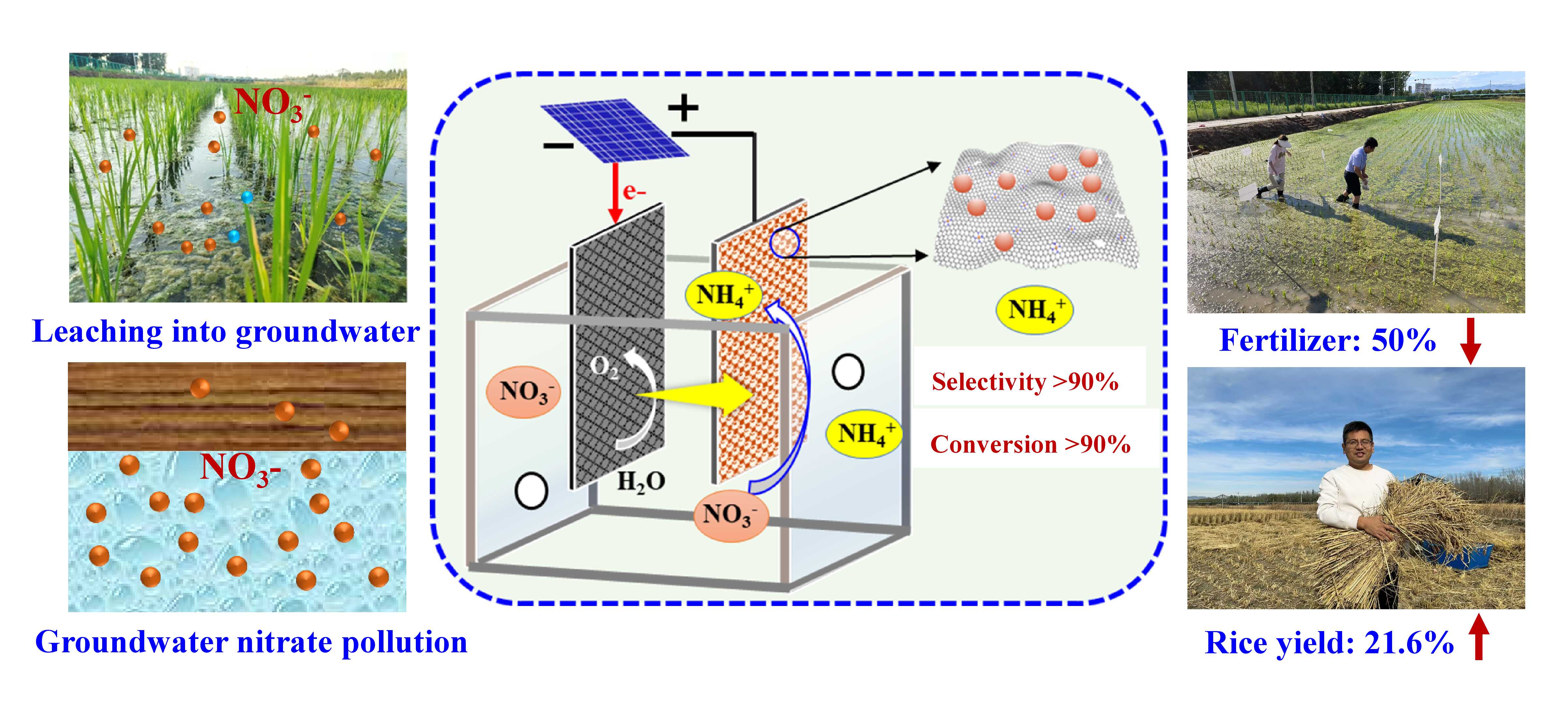
Figure 2 A strategy of converting nitrate into ammonium using electrochemical technology (Image by LIU Chunlei)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

