
Nano-ZnO is a potential catalyst material for carbon dioxide electrocatalytic reduction (CO2RR), but its effective Faraday efficiency (FE) is still below 90% and the current density is less than 300 mA cm-2, which is not enough to meet industrial requirements.
A new study published in Chem Catalysis and conducted by Prof. WU Zhonghua and Dr. XING Xueqing from the Institute of High Energy Physics of the Chinese Academy of Sciences (CAS) and Prof. HAN Buxing from the Institute of Chemistry of CAS reported a kind of ZnO nanorods for electrocatalytic CO2RR, which after 500 °C heat-treatment, achieved the highest oxygen vacancy content, the highest FECO of 98.3%, and a partial current density of 786.56 mA cm-2 in a 3 M KCl electrolyte.
Researcher used a series of advanced detection techniques, such as synchrotron radiation X-ray absorption spectroscopy, X-ray photoelectron spectroscopy, and electron paramagnetic resonance, to confirm the presence of oxygen vacancies. Density functional theory calculations showed that oxygen vacancies can not only change the d-band of ZnO, but also enhance the activity of CO2.
Then, researchers discussed the effect of oxygen vacancy on carbon dioxide electroreduction reaction by using electrochemical characterization methods. The results showed that oxygen vacancies provide excellent kinetic conditions for CO generation and play a key role in accelerating CO2 activation and reducing the energy barrier of CO generation.
This study represents a breakthrough in catalyst materials for the electrocatalytic CO2RR and highlights the potential of low-cost ZnO nanorods rich in oxygen vacancies as robust and efficient catalysts for CO2RR, which are expected to facilitate the development of sustainable energy conversion technologies.
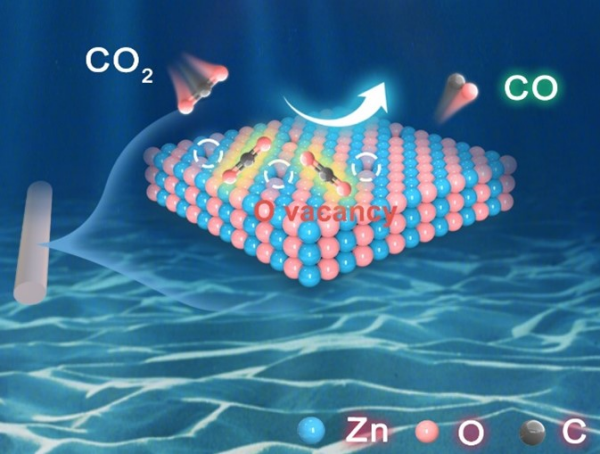
Carbon dioxide electroreduction map on oxygen-rich vacancies, zinc oxide nanorods. (Credit: Chem Catalysis)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

