The electroreduction of carbon dioxide (CO2) to produce value-added multicarbon compounds is an effective way to cut down CO2 emission. However, the low solubility of CO2 largely limits the application of related technology.
Although gas diffusion electrode (GDE) can accelerate the reaction rate, the instability of the catalysts caused by electrolyte flooding hinders further reaction.
Recently, inspired by setaria's hydrophobic leaves, Prof. GAO Minrui's team from University of Science and Technology of China developed Cu catalyst composed of sharp needles which possesses high level of hydrophobicity and stability.
Nature has never failed to be a source of inspiration for scientists. This time, scientists turned to setaria's needle-like leaves to enhance the hydrophobicity of the catalyst in carbon dioxide reduction reaction (CO2RR).
They mimicked the sharp structures on Cu needles to assemble into hierarchical architectures. Such architectures, effectively preventing itself from being wetted just like the setaria's leaves do, enable electrode-electrolyte interface to trap more CO2 as well as help construct robust gas-liquid-solid three-phase boundary that mitigates flooding.
Compared with Cu particles, dendrites hold advantages ranging from stability to productivity.
The predominant hydrophobicity owing to its hierarchical structure doesn't fade appreciably after 10 min of electrochemical operation. Under even more harsh circumstances, like under a constant current density of 300 mA cm-2 for 10 h, the selectivity of the catalyst roughly remains the same with slight loss.
The hierarchical Cu electrode will trap CO2 once they reach the electrode surface, quite similar to the setaria, making mass transfer and accumulation of CO2 possible in practical use.
Furthermore, the selectivity of hierarchical Cu catalyst outstands Cu particles. Cu dendrites can generate the target product, C2+, at a range of applied potentials between -0.53 and -0.68V with overwhelmingly more remarkable C2+:C1+ selectivity of 15.4 at -0.68V over Cu particles.
"The bioinspired hierarchical Cu catalyst effectively mitigates electrolyte flooding by its remarkable hydrophobicity and largely enhances the productivity of CO2RR," said Prof. GAO.
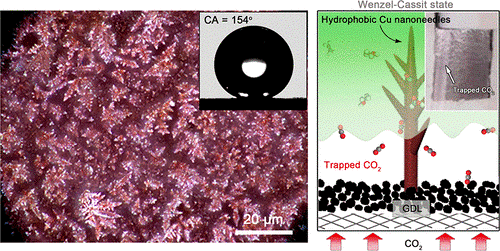
This hierarchical copper structure endows the CO2 reduction electrode with sufficient hydrophobicity to build a robust gas-liquid-solid triple-phase boundary. (Image by NIU Zhuangzhuang et al.)


