
Newsroom
Chloride ion is one of the main ionic components in all organisms. It is well-known that chloride ion plays vital roles for life process. However, the specific roles of chloride ion in protein functions have been rarely reported. So far, chloride ion is known as accessary cofactors of photosystem II, which generate oxygen for the earth, angiotensin-converting enzyme and chloride-dependent α-amylase.
Recently, researchers from the Institute of Oceanology of the Chinese Academy of Sciences have found that a vibrio phospholipase used chloride ion as a core component of its catalytic machinery.
Vibrios are marine bacteria that can infect both marine organisms and human. Phospholipases, which are called thermolabile hemolysins, are important virulence factors, serving as novel drug targets. However, no structural information is available for this class of virulence factors yet. VvPlpA is a phospholipase A2 secreted by Vibrio vulnificus.
In this report, the crystal structure of VvPlpA was solved at a novel resolution. VvPlpA is composed of an N-terminal domain with unknown function and a C-terminal phospholipase domain with a SGNH hydrolase fold, and these two domains are packed closely together. Surprisingly, a chloride ion was identified at the active site region. It substitutes the most common Asp/Glu residue and forms an unusual Ser–His–chloride catalytic triad.
The activity of VvPlpA depended on chloride ion concentration, supporting the important role of chloride ion in catalysis. The VvPlpA structure also shows an inactive oxyanion hole and a large hydrophobic substrate-binding pocket.
This is the first report on the structure of the thermolabile hemolysin family, facilitating drug design against these novel targets. It is also the first illustration of an unusual Ser–His–chloride catalytic triad, which expands the knowledge of the catalytic machinery and the biological role of chloride ion.
The results have been published on the Journal of Biological Chemistry as the cover article.
This paper is also selected as "Editors' Pick", and a accompanied highlighting paper comments that "On the fiftieth anniversary of the discovery of the Ser-His-Asp catalytic triad, perhaps the most unusual variation on the textbook classic is described: An incomplete catalytic triad in a hydrolase is rescued by a chloride ion".

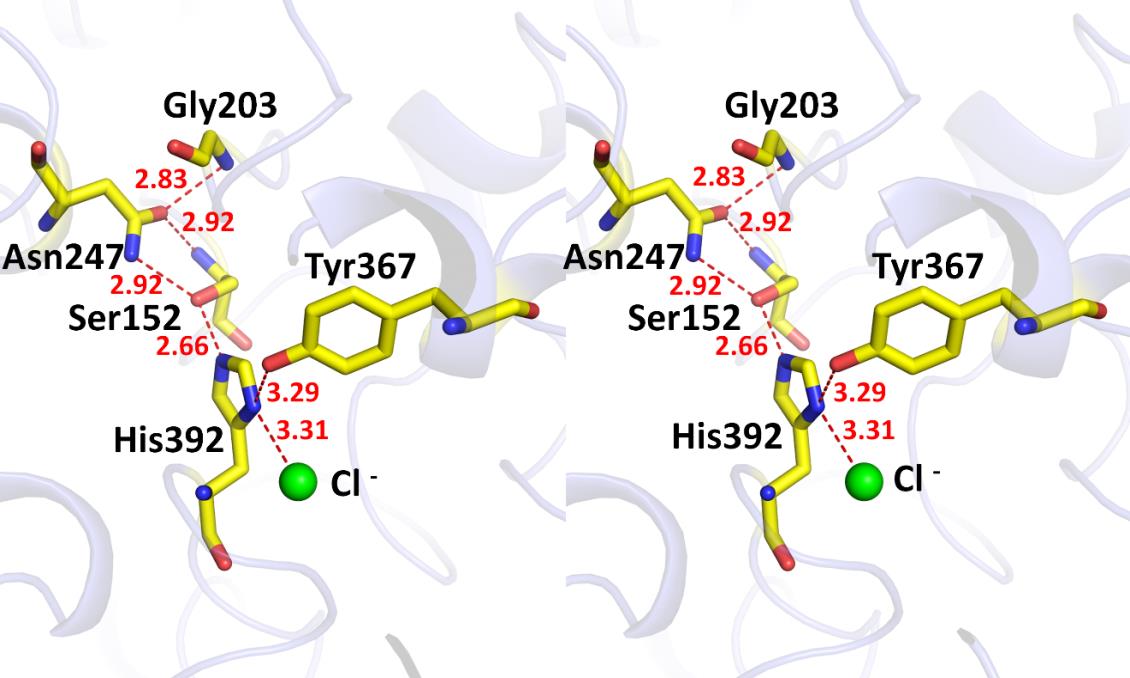
Figure. The overall structure (left panel) and the catalytic site (right panel) of VvPlpA. A chloride ion (green ball) acts as the third component of the unusual Ser–His–chloride catalytic triad. (Image by IOCAS)
