
Human cytochrome P450 2E1 (CYP2E1) has diverse roles in normal physiology, toxicity, and drug metabolism. An enticing property of CYP2E1 is its ability to metabolize ethanol, but the mechanistic basis underlying ethanol oxidation is much less understood.
In a study published in ACS Catalysis, a research group led by Prof. LI Chunsen from Fujian Institute of Research on the Structure of Matter (FJIRSM) of the Chinese Academy of Sciences reported a mechanistic study of CYP2E1-mediated ethanol oxidation by using the combined molecular dynamics (MD) simulations and quantum mechanics/molecular mechanics (QM/MM) calculations.
MD results indicated that Thr303 is of great significance in confining the ethanol substrate within the active site of CYP2E1 through hydrogen-bonding interactions; otherwise, the ethanol substrate continues to tumble until it deviates from the active cavity of CYP2E1.
Meanwhile, QM/MM results showed that the electrostatic interaction between the hydroxyl of ethanol and the oxo of Cpd I could promote ethanol O-H bond cleavage, which thereby makes ethanol O-H bond comparable to ethanol α-C-H bond for hydrogen abstraction process.
Besides, researchers found that the hydrogen-bonding interaction between side chain hydroxyl of Thr303 and ethanol hydroxyl results in a more favorable hydrogen abstraction from the ethanol O-H bond, and the mechanism of CYP2E1-mediated ethanol oxidation includes hydrogen abstraction of O-H bond followed by hydrogen abstraction of α-C-H bond. Due to the endergonic cleavage of O-H bond, hydrogen abstraction of α-C-H bond is the rate-determining step.
These results explain the available experimental data, including the kinetic isotope effect of ethanol α-C-H bond cleavage and the detection of ethoxyl radical in biomimetic ethanol oxidation.
This study highlights a crucial role that the conserved threonine may play in orientating small substrates in the active site of P450 enzymes. The novel effect of threonine provides a new perspective of small molecule activation in P450 catalysis.
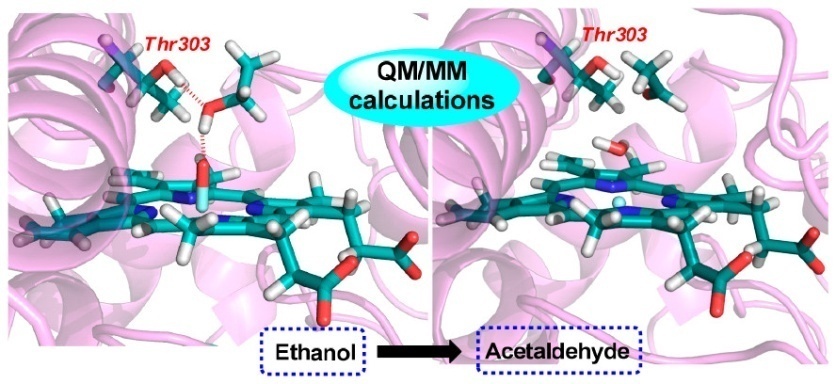
Schematic illustration of the directing effect of Thr303 on ethanol (Image by Prof. LI’s group)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

