
Scientists led by Huaqiang Eric Xu and WANG Mingwei at the Shanghai Institute of Materia Medica (SIMM) of Chinese Academy of Sciences, together with scientists led by ZHANG Yan at Zhejiang University, and scientists led by Jean-Pierre Vilardaga University of Pittsburgh, revealed a near-atomic resolution cryo-electron microscopy (cryo-EM) structure (3.0A) of the human parathyroid hormone type 1 receptor (PTH1R) bound to a long-acting parathyroid hormone (PTH) analog and the stimulatory G protein.
The study, published in Science on April 12, 2019, provided valuable insights into structural basis and dynamics of PTH binding and long-term activation of the receptor, thereby laying a solid foundation for discovering novel therapeutics against osteoporosis, hypoparathyroidism, cachexia and other diseases.
PTH is a classic endocrine hormone identified over 80 years ago. It plays critical and distinct roles in skeletal development, calcium homeostasis, and bone turnover. The functions of PTH are mediated primarily through binding and activation of PTH1R, a member of the class B G protein-coupled receptor (GPCR) subfamily.
As a well-recognized drug target for osteoporosis, PTH1R is highly expressed in bone and kidney cells where it exerts regulatory action in calcium and phosphorus metabolism. Analogs of PTH (such as Teriparatide acetate) are presently used in the clinic to treat osteoporosis.
Previous studies have speculated that endogenous ligand binds and activates class B GPCR through a “two-step” model. The carboxyl terminal of the ligand first binds the extracellular domain (ECD) of the receptor, and then its amino terminus inserts into the hydrophobic pocket of the transmembrane domain (TMD). However, how the ligand dissociates from the receptor remains unknown.
Parathyroid hormone not only activates its cognate receptor quickly but also dissociates rapidly. Using a long-acting agonist, scientists prolonged the residence time of parathyroid hormone on the receptor, and subsequently captured the process of ligand dissociation by means of delicate and meticulous three-dimensional classification during imaging analysis.
They found that the flexible ECD of the receptor remains its inherent dynamic characteristics upon ligand binding and exerts two effects on the helical ligand during the continuous movement. The flexible ECD of the receptor approaches the ligand to produce stress that prompts the ligand unwinding, and moves away from the ligand to weaken their interaction. The combination of the two leads to initial dissociation of the carboxyl end of the ligand.
Besides, the study enhanced the understanding of the molecular recognition mechanism of class B GPCRs. Scientists overcame many technical hurdles such as low PTH1R expression levels, protein instability and difficulties in receptor-Gs complex formation, and eventually determined the three-dimensional structure of a long-acting ligand (LA-PTH) bound to PTH1R-Gs complex.
This structure demonstrated detailed interaction between LA-PTH-bound PTH1R ECD TMD and offers a comprehensive understanding of how PTH1R interacts with a peptide agonist and couples to G protein (Gs). It is the first full-length PTH1R structure in an active state and the first three-dimensional GPCR structure under the long-term activation condition.
Moreover, the study revealed the intracellular cyclic adenosine monophosphate (cAMP) signaling mechanism of PTH1R in a prolonged active state. Due to the high resolution (3.0A) of this complex, scientists unexpectedly discovered the extensively ordered lipid distribution around the TMD which may increase conformational stability of the receptor.
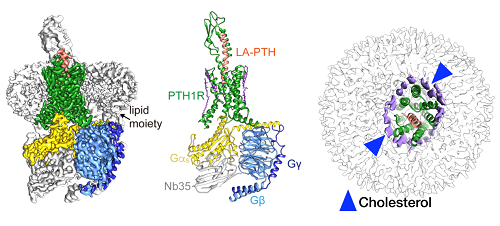
Figure 1: Cryo-EM structure of the human parathyroid hormone receptor-1 signaling complex. (Image by SIMM)
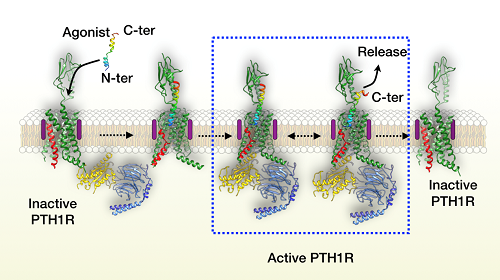
Figure 2: Proposed model of the human parathyroid hormone receptor-1 activation. (Image by SIMM)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

