
Highly-efficient conversion of CH4 to C2H4 with exceptionally high C2H4 yields and C2 selectivity can be achieved in a solid oxide electrolyser. The growth of metal-oxide interfaces at nanoscale with strong interactions in porous electrode scaffolds gives rise to not only improved activity of CH4 activation but also enhanced coking resistance and stability.
In a study published in Nature Communications, a conceptually different process of in situ electrochemical oxidation of methane to ethylene in a solid oxide electrolyser under ambient pressure at 850 °C was reported by a research group led by Prof. XIE Kui from Fujian Institute of Research on the Structure of Matter (FJIRSM) of Chinese Academy of Sciences.
Researchers achieved the highest C2 product selectivity of 81.2% together with the highest C2 product concentration of 16.7% in output gas (12.1% ethylene and 4.6% ethane) in a solid oxide electrolyser and exceptional durability even after 100 hours of operation at high temperatures.
They found that the C2 selectivity remains 41% in the initial pass in the electrochemical process while cycling the output gas for electrochemical oxidation process further enhances the methane conversion to ~80%.
In addition, catalytically active Fe or Ni can be directly doped into the oxide substrate lattice during material synthesis, and then the metal nanoparticles will be developed to anchor to the oxide surface after reduction.
The improvement of the stability and coke resistance of these metal-oxide interfaces would have wide applicability in many other fields of catalysis.
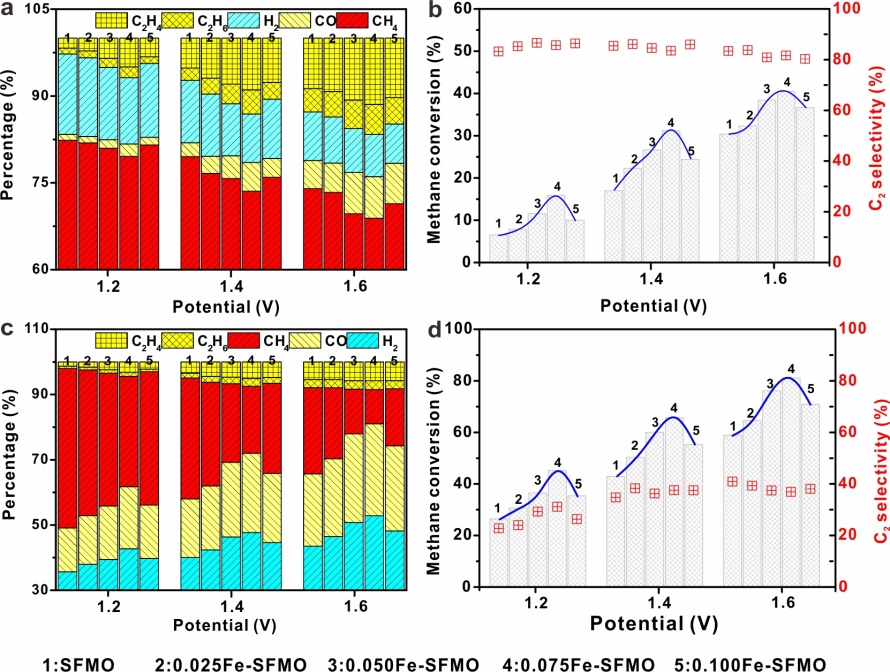
CH4 conversion and C2 selectivity in conjunction with O2 electrolysis.(Image by Prof. XIE’s Group)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

