
In a recent study published in Immunity, Prof. QIAN Youcun’s group at the Shanghai Institute of Nutrition and Health of Chinese Academy of Sciences (CAS) systematically analyzed microbiota changes in lungs of mouse with pulmonary fibrosis, and found that Bacteroides and Prevotella were significantly increased and could secrete Outer membrane vesicles (OMVs) to drive the production of interleukin-17B (IL-17B) to promote the pathogenesis of pulmonary fibrosis.
They also revealed the molecular mechanism of interleukin-17B in regulating the pathogenesis of pulmonary fibrosis, indicating that IL-17B is a potential new target for the treatment of pulmonary fibrosis.
Mucosal microbiota is a hot research area for its important roles in host immunity. It has been proved to be involved in both the immune homeostasis and diseases of various mucosal tissues.
Prof. QIAN’s group has focused on studies about the interactions between mucosal microbiota and interleukin-17 family cytokines for years. On one hand, disorders of microbiota in colon can drive IL-17C to promote the development of intestinal cancer, on the other hand, the commensal bacteria in the intestine can also drive IL-17A production to promote intestinal injury repair. However, the potential roles of pulmonary microbiota in lung tissue remain unclear.
Through high-throughput sequencing, researchers in this study found that there was relatively abundant microbiota in mouse lung tissue under homeostasis, but in the pathological tissues of pulmonary fibrosis, the microbiota was obviously disordered, which was crucial for the pathogenesis of pulmonary fibrosis.
They found that the pulmonary microflora promoted the development of pulmonary fibrosis by inducing the expression of IL-17B. Lung microbiota-depleted mice, germ free mice and IL-17B-deficient mice all showed reduced phenotypes of pulmonary fibrosis.
Further studies identified that Bacteroides and Prevotella bacteria were significantly elevated during pulmonary fibrosis and could promote IL-17B secretion by secreting OMVs.
Using bone marrow transplantation, cell sorting and other experimental methods, researchers found that IL-17B was mainly produced by alveolar macrophages, OMVs out of the dysregulated lung microbiota induced IL-17B expression through Toll-like Receptors TLR2 and TLR4 receptors of alveolar macrophages, and the OMV components such as lipopolysaccharide and lipoprotein were involved in the IL-17B induction.
In addition, they found that IL-17B could directly act on lung epithelial cells to induce the expression of downstream genes to promote the recruitment of neutrophils and the differentiation of Th17 cells and consequently the development of pulmonary inflammation and fibrosis.
In summary, this study reveals the function and mechanism of the local pulmonary microbiota bacteria in regulating lung mucosa-related diseases, especially pulmonary fibrosis, and provides therapeutic targets for clinical treatment of the associated diseases.
Researchers from Harvard Medical School, Shanghai Institute of Biochemistry and Cell Biology of CAS, Shanghai Institute of Immunology, and Shanghai Shuguang Hospital Baoshan Branch also helped with this study.
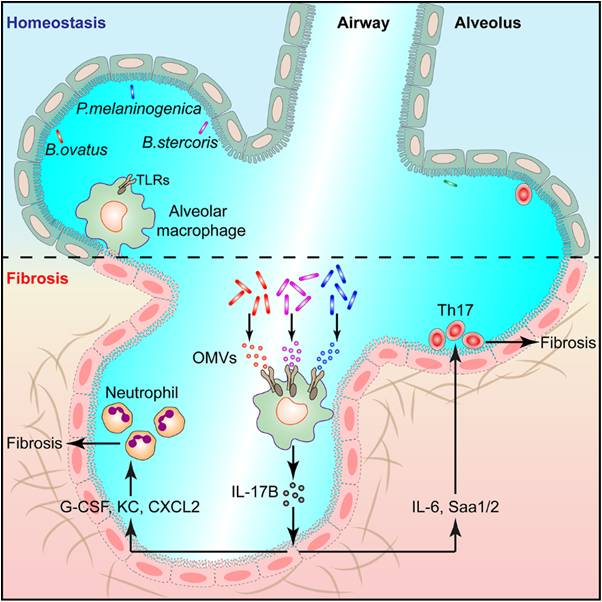
Figure: The model of lung microbiota to promote fibrosis (Image by Prof. QIAN's group)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

