Recently, researchers from Shanghai Institute of Nutrition and Health (SINH) of Chinese Academy of Sciences found that Listeria monocytogenes promotes its own survival by inducing mitochondrial autophagy (mitophagy) in macrophages and identified a new mitophagy receptor NLRX1. The study was published in Nature Immunology.
Mitophagy is a kind of selective autophagy process, which regulates the metabolic state and cell fates by specifically degrading damaged or redundant mitochondria in cells.
Prof. QIAN Youcun's group at SINH has carried out research on nod-like receptors (NLRs) for years. Their previous studies have shown that NLRC5, a member of the NLR family, promotes resistance to infection of Listeria monocytogenes by regulating the expression of major histocompatibility complex protein MHC class I genes, while NLRP3, another NLR member, can be activated by perforin expressed in cytotoxic T cells and promotes anti-tumor immunity. However, the function of NLRX1, the only member of the NLR family located in mitochondria, remains unclear.
To investigate whether bacterial infection activates mitophagy pathways, researchers used intracellular bacteria (Listeria monocytogenes and Salmonella) and extracellular bacteria (E. coli and Citrobacter rodentium) respectively to infect macrophages and systematically analyzed the occurrence of mitophagy. The results showed that intracellular bacterial infections could significantly induce mitophagy in macrophages.
Further studies showed that listeria infection induced calcium influx, mitochondrial damage and mitophagy by secreting hemolysin O (listeriolysin O, LLO).
By means of bioinformatics prediction and RNA interference, researchers found that the NACHT (nucleotide binding and oligomerization domain) domain of NLRX1 contained a conserved autophagy marker LC3 binding motif (LC3-interacting region, LIR) which was required for listeria and LLO-induced mitophagy.
At the physiological functional level, by using NLRX1 gene systemic knockout and macrophage-specific knockout mice, as well as the mitophagy inhibitor Mdivi-1, researchers showed that inhibition of mitophagy led to the accumulation of mitochondrial reactive oxygen species (ROS) in macrophages and the inhibition of listeria survival.
At the mechanism level, they demonstrated that the LRR (leucine-rich repeat) domain of NLRX1 associated with its NACHT domain to maintain a monomeric and resting state, while listeria infection or LLO stimulation led to oligomerization of NLRX1, which facilitated the binding of the LIR motif of NLRX1 to LC3 for mitophagy induction.
This study for the first time demonstrated the critical role of a NLR family member in regulation of mitochondrial homeostasis by identification of NLRX1 as a new mitophagy receptor, and the mechanism exploited by intracellular pathogens to hijack host cell mitophagy, providing new molecular targets and therapeutic strategies for anti-infection therapy.
The study was also assisted and supported by researchers from the University of Toronto, Zhejiang Agricultural and Forestry University and Suzhou University.
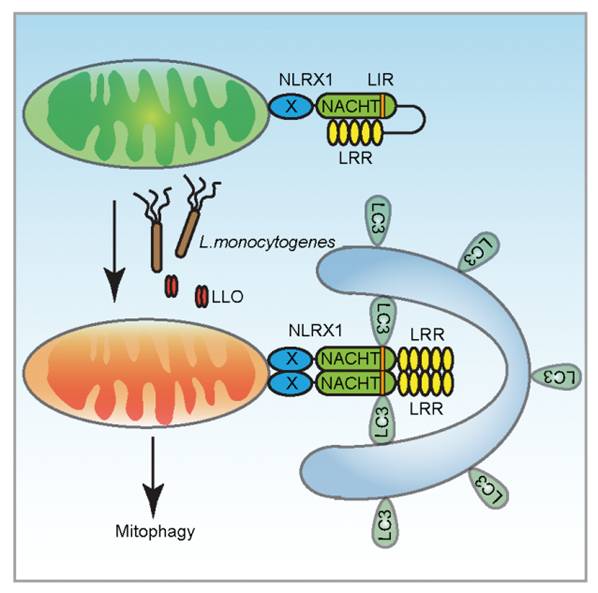
The model for Listeria to hijack host cell mitophagy. (Image by Prof. QIAN's group)





