
Recently, a research team led by Dr. DU Xuemin at the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences created a new shape-morphing scaffold, enabling programmed deformation from a 2D planar cell-laden structure to a well-defined 3D tubular shape, which facilitated the facile 3D endothelialization of small-diameter vascular grafts.
The paper entitled "Programmed Shape-Morphing Scaffolds Enabling Facile 3D Endothelialization" was published in Advanced Functional Materials.
Cardiovascular disease is now the No. 1 cause of death globally according to the World Health Organization, and more than 17.5 million patients die from it every year.
Coronary artery bypass grafting (CABG) is one of most effective approaches for treating severe cardiovascular disease. However, patients undergoing CABG still face the high risks of transplantation surgery and potential complications caused by compliance mismatch.
In recent years, tissue engineering has emerged, holding the promise of constructing functional vascular analogs for treating cardiovascular disease. Nevertheless, 3D endothelialization remains a great challenge for tissue-engineered vascular grafts (TEVGs), particularly small-diameter ones (diameter < 5 mm) suited for CABG, and is the primary problem of TEVGs upon implantation.
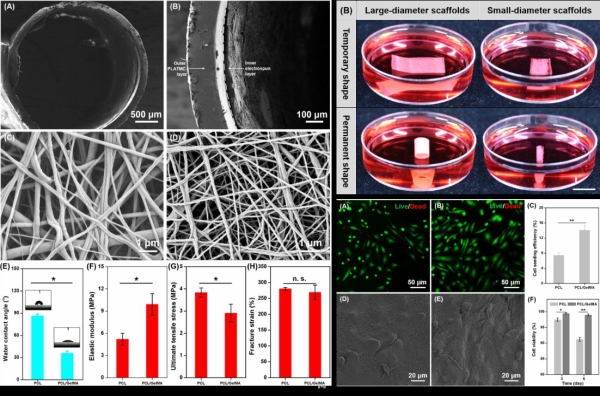
Figure 1. Physical characteristics of shape-morphing scaffolds, and the shape-morphing scaffolds with seeded human umbilical vein endothelial cells (HUVECs). (Image by DU Xuemin)
To address the problem of 3D endothelialization of TEVGs, the researchers designed and developed a novel scaffold, consisting of two layers that combined a shape memory polymer and an electrospun membrane.
By employing the unique shape memory property of the polymer, the scaffold could deform from a 2D planar shape to a well-defined 3D tubular shape at the physiological temperature (37 °C).
The endothelial cells seeded firmly and homogeneously on the electrospun membrane of the planar bilayer scaffold could therefore be conveniently converted to a vascular-like structure of predetermined tubular shape, and a desirable 3D spatial organization of endothelial cells onto the lumen of the scaffold was achieved.
The study found that the 3D cultured endothelial cells on the novel shape-morphing scaffold could form biomimetic cell-scaffold and cell-cell interactions, effectively promoting the formation of a confluent endothelial monolayer and the 3D endothelialization of TEVGs.
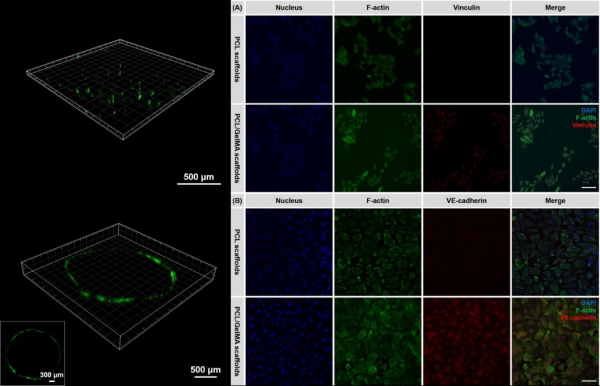
Figure 2. 2D organization of HUVECs (green color, stained by Calcein AM) cultured on the bilayer scaffold, and the immunochemical staining of HUVECs incubated on the different types of shape-morphing scaffolds. (Image by DU Xuemin)
This research not only offers a new method for creating TEVGs that enables facile 3D endothelialization, but also offers a potential in vitro endothelium model for the screening of cardiovascular drugs.
"We hope that the universal strategy developed in this study by combining smart materials and conventional tissue engineering scaffolds can be extended to engineering complex cell-scaffold constructs mimicking the complicated anatomy of various tissues and organs through on-demand programmed deformation," said Dr. DU Xuemin.

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

