
A chemical reaction in the gas phase is a reactive scattering process, after collision between reactants, which is similar to the light scattering, except that reaction products are formed.

The Corona Phenomenon (Image by NASA)
Recently, Prof. WANG Xing'an from the University of Science and Technique of China, Prof. YANG Xueming from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences and their co-workers observed the "corona" phenomenon in a chemical reactive scattering for the first time.
They used the self-setup molecular crossed beam velocity map imaging apparatus, which has the highest resolution in the world. In combination with the study of quantum reactive scattering theory, the underlying mechanism of the "corona" phenomenon was disclosed.
To achieve highest possible resolution in an imaging experiment, scientists used near threshold ionization through (1+1') ionization of the D-atom product. This allowed them to resolve various quantum states of the molecular hydrogen products in the image. Oscillatory structures in the quantum state resolved DCS were clearly observed in the forward scattering direction at the collision energy of 1.35 eV.
In addition, accurate theoretical dynamics analysis by Prof. SUN Zhigang, Prof. ZHANG Donghui of DICP and their co-workers showed that the observed fine structures were mainly caused by a few partial waves near J =28 in the collision, corresponding to an impact parameter of about 2.3 ?.
Intriguingly, the mechanism of these angular oscillations in the forward direction is considered to be very much similar to the corona scattering rings in the atmospheric optics. That is the reason that we call these fine angular oscillations observed as "corona oscillations".
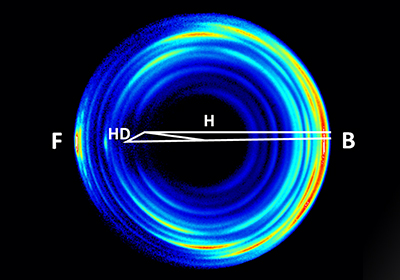
The velocity mapping imaging of the H+HD→H2+D reaction at collision energy of 1.35 eV. (Image by XU Xin et al.)
By capturing the fine angular oscillations, the quantum nature of these oscillations was clearly revealed. This work also demonstrates that velocity map imaging with (1+1’) near threshold ionization is a very powerful experimental technique to investigate the quantum state resolved reaction dynamics of elementary chemical reactions.
This improved experimental technique drives the molecular reaction dynamics study into a true quantum age, since the simple quasi-classical trajectory theory fails to explain such fine experimental measurements any more.
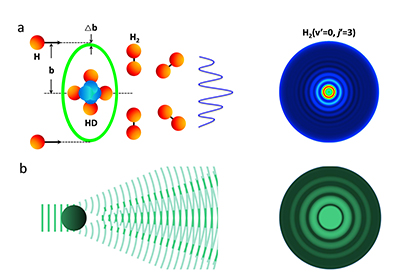
Forward oscillations in a chemical reaction a) and the corona phenomenon. (Image by XU Xin et al.)
Their study entitled "Direct observation of forward-scattering oscillations in the H+HD→H2+D reaction" was published in Nature Chemistry.
The research was supported by the National Natural Science Foundation of China and the Chinese Academy of Sciences.

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

