
Specific populations of neurons in the arcuate nucleus (ARC) of hypothalamus include neurons coexpressing orexigenic neuropeptides agouti-related protein (AgRP) and neuropeptide Y (NPY) along with neurons coexpressing anorexigenic pro-opiomelanocortin (POMC) precursor and cocaine and amphetamine-related transcript (CART), extensively involved in the regulation of appetite, body weight and metabolism.
POMC is a protein expressed and secreted from POMC neurons and cleaved by prohormone convertases to produce α-melanocyte stimulating hormone (α-MSH). The study on the role of POMC neurons in glucocorticoid-induced obesity will provide important theoretical basis for the pathogenesis and treatment of obesity.
In a recent study published in Diabetes, Dr. GUO Feifan’s group at Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences, and the collaborators found that SGK1 expression was decreased in the arcuate nucleus of the hypothalamus, including POMC neurons, following chronic dexamethasone (Dex) treatment, and mice overexpressing constitutively active SGK1 in POMC neurons (PSOE) prevented from Dex-increased adiposity.
The researchers demonstrated that acute Dex treatment induces SGK1, and SGK1 expression was decreased in ARC POMC neurons of chronic Dex-treated mice. The difference in SGK1 expression under acute or chronic Dex treatment may be caused by differences in glucocorticoid receptor (GR) activity under different conditions.
To study the role of SGK1 in POMC neurons, mice with development or adult-onset SGK1 deletion in POMC neurons (PSKO) were then produced by researchers. As observed in Dex-treated mice, PSKO mice exhibited increased adiposity and decreased energy expenditure. Consistently, PSOE mice had the opposite phenotype and prevented from Dex-increased adiposity.
Finally, Dex decreased hypothalamic α-MSH content and its precursor Pomc expression via SGK1/Forkhead box O3 (FOXO3) signaling and intracerebroventricular injection of α-MSH or adenovirus-mediated FOXO3 knockdown in ARC largely reversed the metabolic alterations in PSKO mice.
These results demonstrated that POMC SGK1/FOXO3 signaling mediates glucocorticoid-increased adiposity, providing new insights into mechanistic link between glucocorticoid and fat accumulation and important hints for possible treatment targets for obesity.
This study was funded by the National Natural Science Foundation of China, Shanghai Science and Technology Commission and Chinese Academy of Sciences.
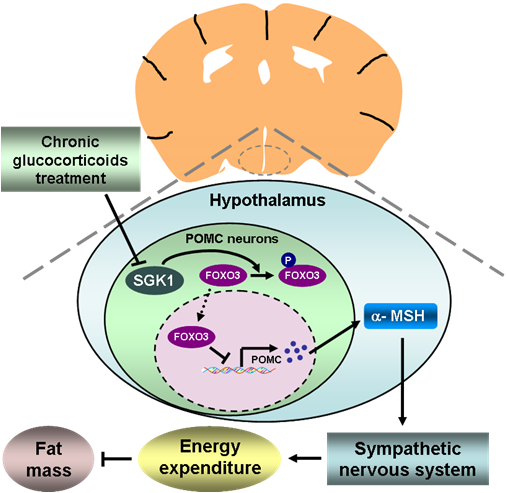
Figure: SGK1/FOXO3 signaling in hypothalamic POMC neurons mediates glucocorticoid-increased adiposity (Image by Dr.GUO Feifan’s lab)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

