
Inflammasomes are recently identified as cytosolic multi-protein complexes mediating specific proinflammatory signals such as maturation of IL-1β, IL-18 and processing Gasdermin D to provoke a specialized cell death, pyroptosis. They have been involved in gut homeostasis and inflammatory pathologies. Among many different inflammasomes, NLRP3 stands out for its complicated activation mechanisms, as well as its clinical significance.
In humans, dysregulation of inflammasome signalling by gain-of-function mutations in the coding region of NLRP3 results in a group of autoinflammatory diseases called cryopyrin-associated periodic syndromes. Mice carrying the homologous mutation in NLRP3 (NLRP3-R258W) show similar inflammation in skin and joints, but retained a healthy intestine, which indicates an unknown pathway for NLRP3-R258W to maintain gut homeostasis despite its hyperactivity.
In a recent study published in Nature Communications, MENG Guangxun's group at Institute Pasteur of Shanghai of Chinese Academy of Sciences, in cooperation with Prof. ZHAO Liping’s lab at Shanghai Jiaotong University, found that the NLRP3-R258W mice are not only healthy with their intestines, but also are with stronger resistance to dextran sulfate sodium (DSS) colitis and colorectal cancer.
The mutation led to a distinct gut microbiota with a sparingly interconnected co-abundance network, and nourished functional bacteria capable of inducing Tregs, which mediates neutralization of intestinal inflammation. Lack/inhibition of Tregs would lead to spontaneous colitis/loss of resistance to induced acute colitis.
Mechanistically, the microbiota was reshaped via increased local antimicrobial peptides boosted by the enhanced IL-1β but not IL-18 production from lamina propria mononuclear phagocytes containing mutated NLRP3.
This study for the first time revealed how NLRP3 functions in the intestine and how it cooperates with gut microbiota to harnessing Tregs to maintain intestinal homeostasis. It highlighted the importance of NLRP3’s contribution to the gut diseases and homeostasis, providing potential molecular targets for future treatments of intestinal disorders.
The study was funded by grants from Natural Science Foundation of China, National Key Basic Research programs of China, as well as Strategic priority program and International partnership program of Chinese Academy of Sciences.
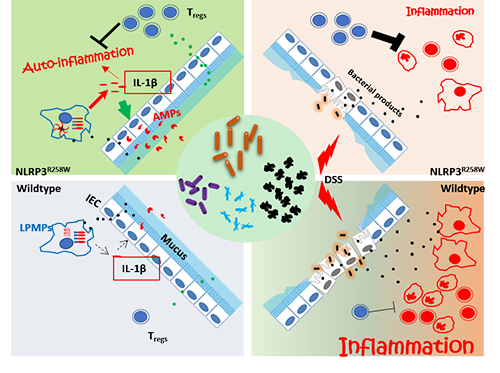
Figure: Remodeling of the gut microbiota by hyperactive NLRP3 inflammasome induces regulatory T cells to maintain intestinal homeostasis (Image by the group)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

