
Plasmonic nanostructures which are characterized by strong light absorption through the excitations of collective free electron oscillations are known as surface plasmon resonance (SPR). Such nanostructures have recently shown great potential in solar energy conversion.
The plasmonic hot electrons in water reduction have been extensively studied, however, how the plasmonic hot holes participate in the water splitting reaction exactly has not been well understood. In particular, it's still illusive that where the plasmonic hot holes participate in water oxidation.
Recently, the research group led by Prof. LI Can at Dalian Institute of Chemical Physics (DICP) of Chinese Academy of Sciences identified the water oxidation reaction sites in plasmonic photocatalyst Au/TiO2 and fabricated a visible-light-induced overall water splitting system based on surface plasmon effect. This work has been published in J. Am. Chem. Soc.
Researchers demonstrated that the plasmon-induced water oxidation reaction takes place at the reaction sites localized at the interface between Au and TiO2. The reaction sites are positioned by photodeposition together with element mapping by electron microscopy, while the distribution of holes is probed by surface photovoltage imaging with Kelvin probe force microscopy. Density functional theory also corroborates these findings by revealing the promotion role of interfacial structure (Ti-O-Au) for oxygen evolution.
Based on the above results, they successfully assembled an artificial Z-scheme photosynthesis system with redox ions for overall water splitting under visible light illumination. What’s more, the group developed an all-solid plasmon-induced Z-scheme system for overall water splitting, which is more challenging to achieve overall water splitting in an all-solid artificial photosynthesis system. This work has been published in J. Catal.
The study was supported financially by National Natural Science Foundation of China, 973 National Project (Grant No. 2014CB239400), the Strategic Priority Research Program of the Chinese Academy of Sciences, and the DICP Innovation Foundation.
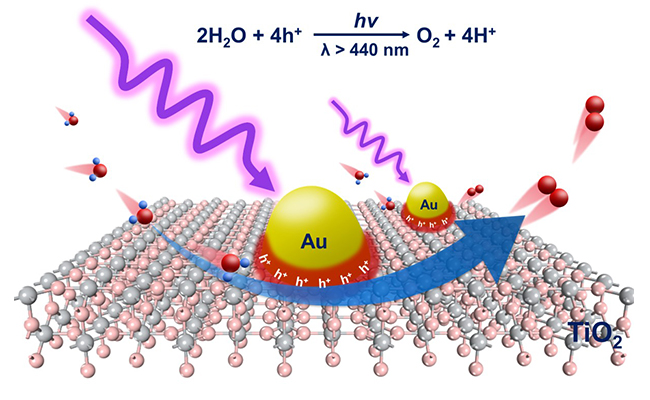
Gold-semiconductor interface is identified as reaction sites for water oxidation (Image by WANG Shengyang)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

