
Many RNA viruses encode helicases to aid their RNA genome replication and transcription by unwinding structured RNA. Being naturally fused to a protease participating in viral polyprotein processing, the NS3 helicases encoded by the Flaviviridae family viruses are quite unique. Therefore, how these two enzyme modules coordinate in a single polypeptide is of particular interest.
Recently, in cooperation with Prof. PAN Zishu from Wuhan University, the research group led by Prof. GONG Peng from Wuhan Institute of Virology of the Chinese Academy of Sciences reported a previously unidentified conformation of pestivirus NS3 in complex with its NS4A protease cofactor segment (PCS). This conformational state is related to the protease cis-cleavage event and is optimal for the function of helicase.
In this study, through a flexible linker and solved its crystal structure at 2.35 Å resolution, scientists have crystallized the full-length CSFV NS3 with its NS4A PCS covalently tethered to its N-terminus .
The structure provides structural insights into pestivirus NS3-NS4A cis-cleavage and protease substrate recognition, allowing them to find evolutionary linkages between pestivirus NS3 protease and its hepacivirus and flavivirus counterparts.
More importantly, the structure reveals a previously unidentified intra-molecular interface between the protease and helicase modules that may play key roles for the function of both enzymes. In vitro helicase assays and virological data further validated the functional importance of this interface and its relevance to the RNA unwinding function of the helicase.
The structural and functional analyses in this work pave a way to the further understanding of how this natural protease-helicase fusion protein works in harmony and how its different conformational states may play distinct roles to achieve versatile functions.
The results have been published in Journal of Virology entitled "The uncoupling of protease trans-cleavage and helicase activities in the pestivirus NS3".
This work was supported by the National Key Basic Research Program of China and the National Natural Science Foundation of China.
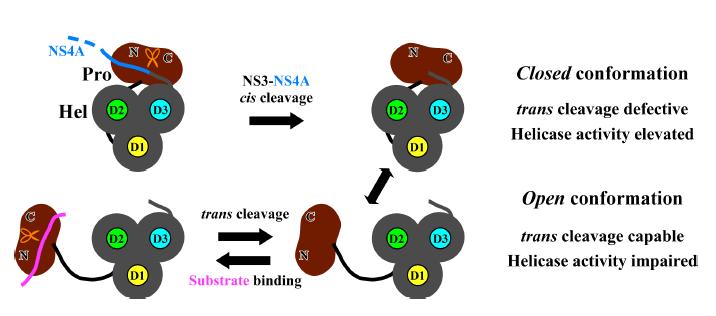
A working model describing the relationship between the pestivirus NS3 conformational states and the protease/helicase activities. (Image by GONG Peng)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

