
Single molecule fluorescence resonance energy transfer (smFRET) is a powerful method to reveal the conformational heterogeneity and dynamics of biomacromolecules, which is crucial for understanding the relationship between structure and function.
However, the most widely applied cysteine-maleimide reaction has limitations for the fluorescence labeling of homodimeric proteins. In previous studies, methods such as subunit exchange or truncation were usually used to obtain heterodimers or monomers for FRET labeling, but these methods have obvious limitations.
In this study, Prof. Sarah Perrett and Dr. WU Si from Institute of Biophysics (IBP) of Chinese Academy of Sciences based on a co-expression system presented a generic method for site-specific incorporation of a single donor-acceptor dye pair into any desired position in a dimeric protein.
The yeast prion protein Ure2 forms a highly stable homodimer and does not undergo subunit exchange on a measurable timescale. Researchers therefore applied this co-expression strategy to label Ure2 with sets of FRET dye pairs introduced at different sites within the dimer.
By measuring the distances between the dye pairs, single molecule FRET provided insight into the conformation of the intrinsically disordered prion domain of Ure2 in the context of its globular C-terminal domain.
The results revealed the spatial position and the collapsed nature of the prion domain, providing clues to the inter-domain relationship.
This labeling method provided a new approach to probe the structure or conformation of intrinsically disordered proteins (IDPs) that are not monomeric, and has potential applications in the smFRET study of other dimeric proteins that have important biological functions.
The study was published as the inside front cover article in ChemComm. It was funded by the National Natural Science Foundation of China and the 973 Program of the Ministry of Science and Technology.
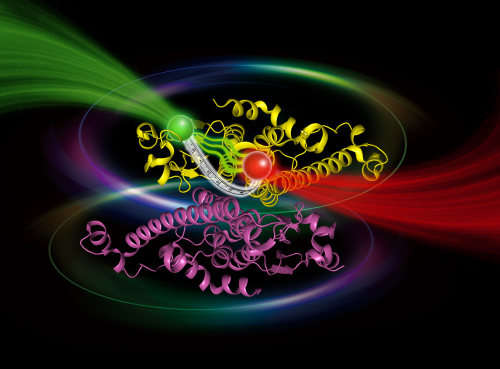
Figure: The co-expression strategy used to obtain labeled heterodimers of Ure2, in order to study the conformation and dynamics of the Ure2 prion domain by single molecule FRET. (Image by IBP)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

