
Allostery is the process by which biological macromolecule complexes transmit the effect of binding at one site to another, often distal, functional site, allowing for regulation of activity. It exists widely in the regulation of many important biological processes, such as enzyme activity, receptor activity in signal transduction pathway, ion-channel, and gene expression.
Allosteric regulation is usually described by equilibrium models. The regulation of the bacterial flagellar switch, as a basis for bacterial motility, is a typical allosteric regulation process. There is a long standing controversy in the last decades on whether this is an equilibrium process or a non-equilibrium one with external energy input.
The research group led by Prof. YUAN Junhua and ZHANG Rongjing from University of Science and Technology of China (USTC) has discovered non-equilibrium effect in the regulation of the bacterial flagellar switch by systematically studying the motor switching dynamics under various experimental conditions.
They further developed a non-equilibrium model to explain the energy input mechanism. Their findings resolved the long-standing controversy.
This work has been published in Nature Physics entitled "Non-equilibrium effect in the allosteric regulation of the bacterial flagellar switch", as well as a "News and Views" article titled "Biomolecular switches: Driven to peak" on the same issue.
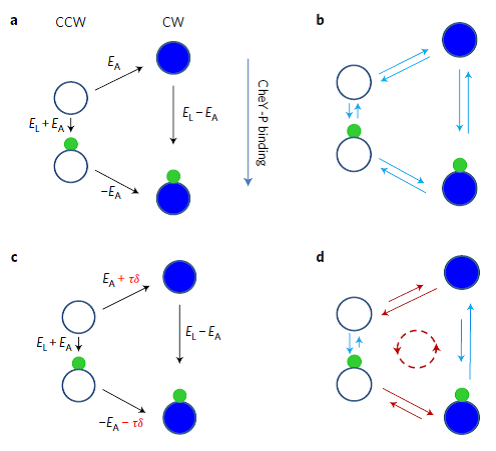
Modelling the flagellar switch. a, Free energy diagram of the four states of a subunit of the flagellar switch in the equilibrium conformation spread model. Each subunit can be in CCW or CW states, and has a single binding site for CheY-P. b, Detailed balance of the transition rates is satisfied between each pair of states in the equilibrium model. c, Non-equilibrium effect due to the motor torque is added to the equilibrium model to increase the free energy separation between CW and CCW states. d, Non-equilibrium effect leads to breakdown of detailed balance between CW and CCW states, resulting in net rate flux (dashed line). (Image by Nature Physics)
The rotary bacterial flagellar motor tunes the type of bacteria motion, which is a random walk pattern alternating between runs and tumbles.
In this article, it is pointed out that the bacterium runs when all of the flagellar motors on a cell rotate in a counterclockwise (CCW) direction, and tumbles when one or more motors rotate in a clockwise (CW) direction.
The study of these two states interval distributions can help to understand motor-switching dynamics better. However, the resulting CCW or CW interval distributions are sometimes different among the experiments.
In order to determine the cause behind those differences and investigate the mechanism of motor switching, the authors systematically study motor switching under various load conditions from high to zero load, under different proton motive force (pmf) conditions and varying the number of torque-generating units (stators) and they find the signature of a non-equilibrium effect between CCW and CW states.
This non-equilibrium mechanism leads to a force-sensing mechanism for the bacterium. Although only about 0.2% of the energy input for the motor is utilized for allosteric regulation, it nevertheless increases the motor sensitivity greatly. Similar mechanisms are expected to be found in other protein complexes.
To sum up, the motor switch dependence on load, pmf, and number of stators described in this article can be consistently explained in terms of the dependence on motor torque and the results also resolve the previous discrepancy among different experiments.
This work was supported by National Natural Science Foundation of China, Fundamental Research Funds for the Central Universities, and Anhui Natural Science Foundation. Prof.s YUAN Junhua and ZHANG Rongjing are supported by the "1000 Youth Talent Program".

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

