
The C25 sesterterpenoids, derived from five isoprene units, are widely distributed in marine organisms, terrestrial fungi, lichens, higher plants, insects, etc. Many possess intriguing skeletons and exhibit interesting bioactivities. The sesterterpenoids only constitute a relatively small group among terpenoids.
So far, only several sesterterpene synthases (sesterTPSs) mainly from fungi have been reported. Interestingly, they are responsible for the biosynthesis of a series of sesterterpenes with diverse carbon skeletons. However, to date, sesterTPSs originated from plants have never been disclosed.
Prof. WANG Yong's group at Institute of Plant Physiology and Ecology of Chinese Academy of Sciences (CAS), collaborating with Prof. WANG Guodong’s group at Institute of Genetics and Developmental Biology of CAS, identified two plant sesterTPSs in the genome of A. thaliana by coexpressing geranylfarnesyl diphosphate synthase (GFPPS) with the two GFPPS-linked AtTPS18 and AtTPS19 in E. coli, and further obtained their functional products. The study was published online in Organic Letters.
AtTPS18 is responsible for the biosynthesis of the new tricyclic sesterterpene (+)-thalianatriene with the unique 11-6-5 fused ring system, while AtTPS19 functionally produces the new pentacyclic sesterterpene (-)-retigeranin B with the characteristic 5-5-5-6-5 fused ring system.
Interestingly, the trans-angular bond between the (E,E)-∆3(20),6,10 cycloundecatriene and the cyclohexane of (+)-thalianatriene is rarely formatted at C-2 to C-12, difference from C-1 to C-2 of the known related 11-6-5 carbon skeleton, as in stellata-2,6,19-triene. The structure elucidation of (+)-thalianatriene is challenging, because the conformers in (+)-thalianatriene caused by the inversion of the double bonds around the macrocycle are too complex to be discriminated even in -40 oC NMR measured condition.
A major epoxy (+)-6,10-diepoxy-thalianaene can be purified, which greatly simplifies its conformers. The variable-temp NMR measurement indicates that (+)-6,10-diepoxy-thalianaene exists as an indiscernible mixture of two conformers in CDCl3 solution in a ratio of about 2:1 at or below ordinary probe temp. The most stable conformer is assigned as the UUU (chair-twist) form, while the less populated conformer attributes to the UUD (chair-chair) orientation. X-ray analyses shows that only the most stable, predominant conformer UUU, exists in the solid state.
Finally, the structure of (+)-thalianatriene is successfully determined and a plausible biosynthesis mechanism of (+)-thalianatriene is proposed. This is the first report of the discovery of the sesterTPSs from plants and their functional products.
This study was supported by Chinese Academy of Sciences, National Natural Science Foundation of China, and Shanghai Committee of Science and Technology.
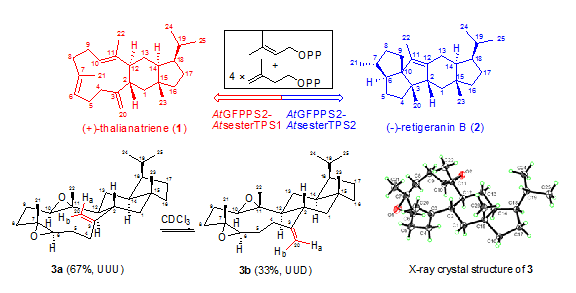
Figure: Sesterterpenes obtained in this study (Image by WANG Yong's group)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

