
Nonlinear optical (NLO) material is one of the important parts of solid-state lasers. It is largely used in laser frequency conversion, optical parameter oscillators, other optical and photonic devices.
Designing and synthesizing a deep UV NLO material requires crystallographic non-centrosymmetry, a wide UV transparency range, a large second-harmonic generating coefficient, moderate birefringence, chemical stability and resistance to laser damage, and ease in the growth of large high-quality single crystals. For example, β-BaB2O4, LiB3O5 and CsLiB6O10 are successful example of NLO material. Recently, metal phosphates have attracted considerable interests for designing new DUV NLO materials.
A research group led by Prof. PAN Shilie at Xinjiang Technical Institute of Physics & Chemistry (XTIPC) of the Chinese Academy of Sciences developed three new NLO crystals APb2(PO3)5 (A = K, Rb and Cs).
The three compounds are isostructural and belong to the non-centrosymmetric space group of Pn (No. 7). The structures of APb2(PO3)5 (A = K, Rb and Cs) exhibit a 3D network which consists of the PbOn (n = 6, 8), KO7/RbO8/CsO10 and PO4 units.
Researchers found UV-vis-NIR transmittance spectrum result shows that KPb2(PO3)5 has a short deep-UV cutoff edge of 177 nm. It is the shortest UV cut-off edge among Pb-containing NLO crystals.
Second harmonic generation (SHG) result indicate that KPb2(PO3)5 and RbPb2(PO3)5 are display moderate powder SHG intensities (0.5 × KDP and 0.3× KDP, respectively) in type I phase matching behaviors. Compared with RbBa2(PO3)5, KPb2(PO3)5 has a noticeable enhancement in birefringence (experimental value of 0.03 at 589.3 nm).
To better understand the blue-shift mechanism and enhancement of birefringence in KPb2(PO3)4, researchers employed the theoretical calculations by density functional theory.
As a result, there is almost no hybridization between Pb-6p and O-2p orbitals at the top of valence bands, and therefore, the stereochemistry activity of the lead atom in KPb2(PO3)5 is weak according to the stereochemistry activity mechanism, which causes a blue-shift of the band gap in KPb2(PO3)5.
The result was published in J. Mater. Chem. C as a front cover. This article is part of themed collection: 2016 Journal of Materials Chemistry C Hot Papers.
This work was supported by the National Natural Science Foundation of China.
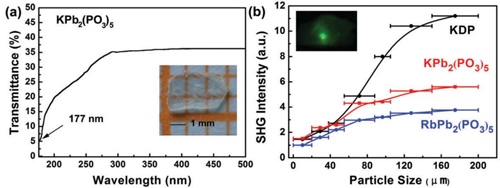
Figure1:The DUV transmittance spectrum and the photograph of the KPb2(PO3)5 crystal plate. (b) NLO properties of KPb2(PO3)5 and RbPb2(PO3)5 with commercial KDP as a reference(Image by XTIPC)

Figure 2:The front cover of the article(Image by XTIPC)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

