
Among the different clean and renewable energy systems that are currently available, direct methanol fuel cells (DMFCs) have been extensively studied in recent decades because of their simple structure, high power densities, high energy conversion efficiencies, low operation temperatures and low pollutant emissions. Therein, methanol is directly oxidized by oxygen from air into water and carbon dioxide to generate electricity.
As known, Pt-based materials are widely used as anode electrocatalysts for driving methanol oxidation reactions. However, the successful commercialization of DMFCs was greatly hampered by the high manufacturing cost, slow reaction kinetics of Pt-based catalysts, and the poisoning of Pt catalysts by CO-like species during the process of methanol oxidation.
Considering the shortcoming of Pt-based catalysts, a research group in Key Laboratory of Materials Physics and Anhui Key Laboratory of Nanomaterials and Nanotechnology, Institute of Solid State Physics, Hefei Institutes of Physical Science, Chinese Academy of Sciences, proposed a simple and green strategy to fabricate a two dimensional Pt/SnO2/rGO hybrid nanocomposite (NC) based on laser ablation in liquids (LAL) technique combined with a facile photo-assisted in situ reduction method (Figure 1).
In the present effort, LAL-induced fresh SnOx colloids showing a low valence state of cation Sn and poor crystallization were first dispersed on the surface of GO sheets.
After aging treatment at room temperature, GO sheets in situ evolved into rGO sheets, whereas the poorly crystalline SnOx NPs were simultaneously converted into well-crystallized SnO2 NPs and uniformly distributed on rGO sheets as well.
Expectedly, the high distribution of SnO2 NPs on rGO sheets would result in high dispersion of Pt catalysts. Under the UV-light irradiation, the photo-excited electrons migrated from the valence band of SnO2 NPs to the conduction band, leaving the holes in the valence band.
The PtCl62- absorbed on the surface of SnO2 was then in situ reduced to Pt° atom by the photo-excited electrons. Then, many reduced Pt atoms aggregated into ultrafine Pt NPs, which were uniformly anchored on the surface of SnO2.
Several benefits of this work are inspired. Firstly, the photo-excited electrons from SnO2 NPs were used as clean reducing agents and no organic surfactant was introduced during the whole synthetic process, then, the reduction reaction of Pt occurred on the surface of SnO2, thereby leading to the ultrafine Pt NPs (1-2 nm) in situ anchoring on the surface of SnO2 NPs.
Moreover, the ultrafine Pt NPs distributed on the surface of SnO2 evenly and favored the increase of active surface per weight of Pt, which are beneficial to reduce the manufacturing cost of Pt catalysts.
Otherwise, the metal oxides possess a great capacity to store and release oxygen, which would play an important role in further oxidizing the CO-like species; and the as-prepared Pt/SnO2/rGO catalysts present higher catalytic activity and better long-term performance toward methanol oxidation compared with the Pt/rGO catalysts.
Thus, as anode catalysts toward methanol oxidation, these ternary hybrids displayed significantly reduced over-potential, greatly enhanced catalytic activity, and notably improved long time endurance compared with the conventional Pt catalysts supported on rGO sheets (Figure 2).
Their research was published in journal of Nano Energy with title Photo-excited in situ loading of Pt clusters onto rGO immobilized SnO2with excellent catalytic performance toward methanol oxidation.
This study was sponsored by the National Basic Research Program of China, the National Natural Science Foundation of China.
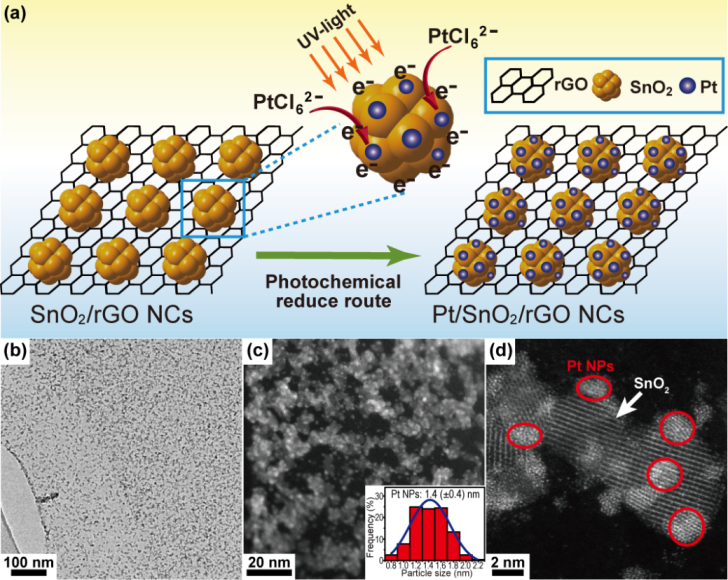
Figure 1. Pt0.89/SnO2/rGO catalysts: (a) Illustration for the synthesis of Pt/SnO2/rGO hybrid ternary nanocomposite; (b) Low-magnification TEM image; (c-d) HAADF-STEM images; the inset of (c) is the particle size distribution histogram of Pt NPs. (Image by WU Shouliang)
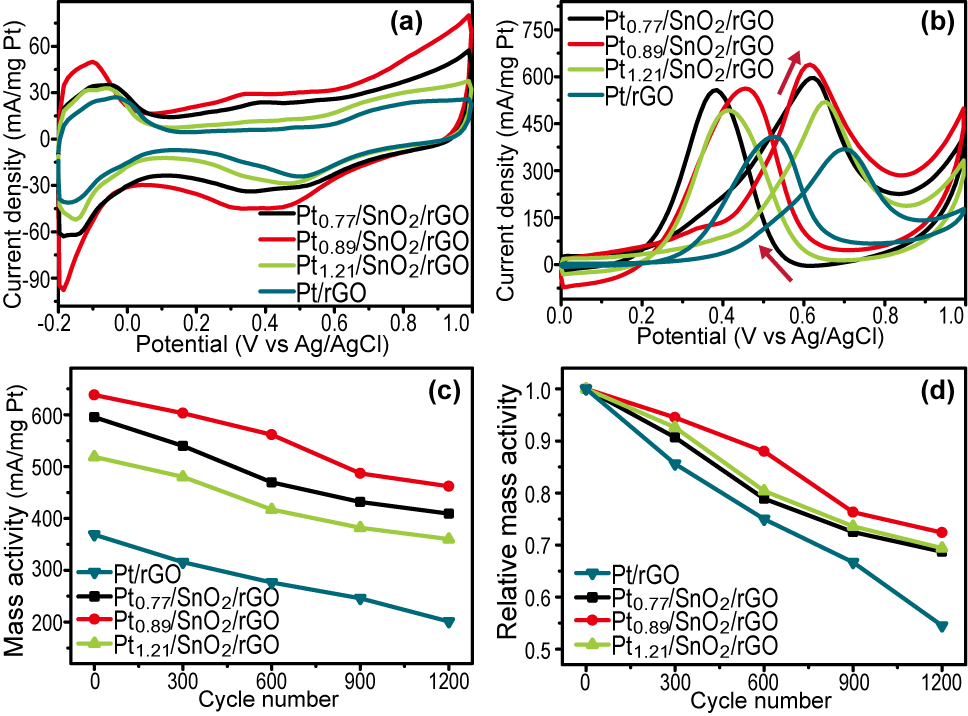
Figure 2. CV curves of GC electrodes modified with different catalysts measured in (a) 0.5 M N2-saturated H2SO4 and (b) 0.5 M H2SO4 + 0.5 M CH3OH; (c-d) the catalysis stability of different catalysts for methanol oxidation. (Image by WU Shouliang)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

