
Eantioselective C-H functionalization of sp3-hybridized carbon-hydrogen bonds in complex organic molecules is of great importance in modern synthetic chemistry, especially for asymmetric synthesis.
Recently, the research team led by LIU Guosheng from the Shanghai Institute of Organic Chemistry (SIOC) of Chinese Academy of Sciences and Shannon S. Stahl from the University of Wisconsin-Madison developed an efficient approach to directly convert hydrocarbons into enantiomeric enriched nitriles, which are privileged in pharmaceuticals and agricultural chemicals.
Inspired by enzyme-catalyzed C-H bond hydroxylation and halogenation, researchers discovered a copper/bisoxazoline system to achieve enantioselective functionalization of benzylic C–H bonds with high efficiency.
However, distinct from "radical rebound" in enzymatic catalysis, the present protocol proceeded via a “radical relay” process, and exhibited excellent stereo-control on the reactive radical intermediate. All these reaction underwent smoothly at room temperature with the benzylic substrates limiting reagent with excellent chemo-, regio- and enantioselectivity (typically90% to 99% ee).
Meanwhile, researchers observed broad substrate scope and excellent functional group compatibility. These features provide key foundation for the pursuit of other highly selective C-H oxidation reactions.
This study has been published in Science and was supported by National Basic Research Program of China and the National Natural Science Foundation of China.
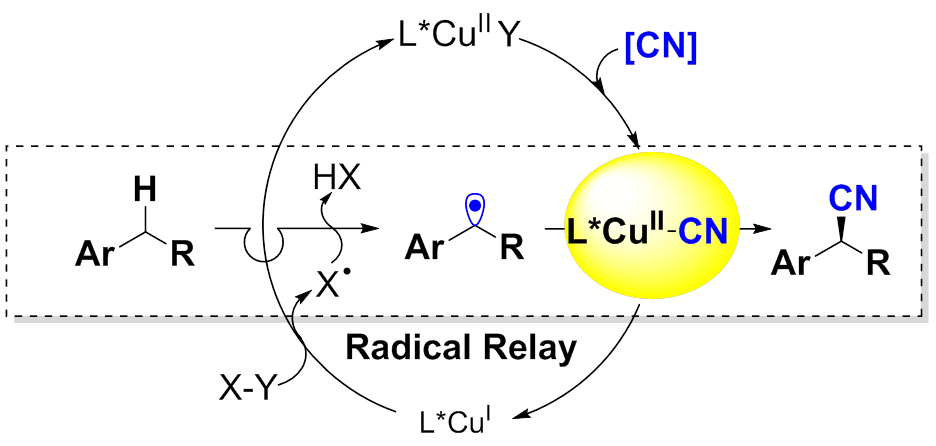
Figure: Radical Relay Process for Enantioselective C-H Cyanation (Imaged by LIU Guosheng)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

