

New research into the ratio of elements in Earth's mantle suggests that the planet's carbon might have come from a collision with a planet embryo around 4.4 billion years ago.
The early Earth could have been hit by a Mercury-like planet embryo deep in its past, letting the planet keep hold of the carbon necessary for life, a new study suggests.
The Experimental Petrology Rice Team — who study the origin, composition and structure of rocks at Rice University in Texas — re-created conditions inside Earth in the lab to help solve a long-standing mystery: how carbon-based life could have developed on Earth when early carbon would have boiled away or sunk to the planet's core.
"The challenge is to explain the origin of the volatile elements like carbon that remain outside the core in the mantle portion of our planet," Rajdeep Dasgupta, a co-author on the new study and petrologist at Rice, said in a statement.
Dasgupta's group previously investigated how carbon would have fared on the early, molten Earth: Even if the carbon didn't vaporize into space, it would have been drawn into the metallic core after bonding with iron-rich alloys, Dasgupta said.
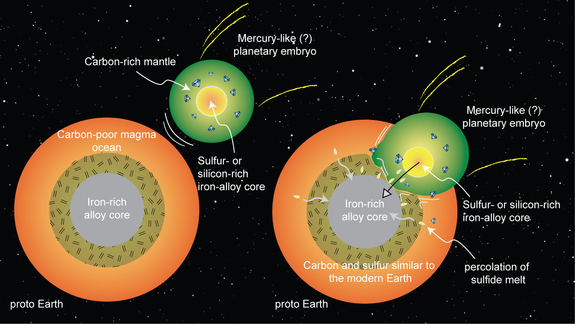
How Earth's merger with a potentially Mercury-like planetary embryo might have transferred carbon and sulfur to Earth's mantle.
Researchers have postulated that carbon and other elements now present on Earth came from meteorites or comets hitting the planet.
"One popular idea has been that volatile elements like carbon, sulfur, nitrogen and hydrogen were added after Earth's core finished forming," the study's lead author, Yuan Li, now a staff scientist at Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, said in the statement. "Any of those elements that fell to Earth in meteorites and comets more than about 100 million years after the solar system formed could have avoided the intense heat of the magma ocean that covered Earth up to that point.
But the real-life proportions of elements on Earth don't seem to match up with comets and meteorites: "The problem with that idea is that while it can account for the abundance of many of these elements, there are no known meteorites that would produce the ratio of volatile elements in the silicate portion of our planet," he added, referring to a main component of rocks in Earth's mantle, the 1,800-mile-deep (2,890 kilometers) soup of magma and rock between the crust and core.
The researchers' experimental setup re-created the high-temperature and high-pressure interior of Earth by squeezing rocks with hydraulic presses. This method can emulate conditions about 250 miles (400 km) below Earth's surface, in the planet's mantle. The investigators decided to test whether the elements silicon or sulfur — found in the cores of Mercury and Mars, respectively — had any impact on the amount of carbon that would be bound up within Earth's core.
The scientists found that when sulfur bonded with iron under intense pressure — like that in Earth's core — it kept carbon from bonding with the molecules in the core as strongly, the researchers said in the statement. (In the paper, published in the journal Nature Geoscience on Sept. 5, the scientists said that "in reduced or sulfur-rich bodies, carbon is expelled from the segregating core.") In that scenario, the carbon could remain higher up, in Earth's mantle, and be available later on for the development of life instead of being locked away, the study said.
The researchers then compared the concentrations of carbon that emerged during the experiments with the concentrations of elements in Earth's actual mantle.
"One scenario that explains the carbon-to-sulfur ratio and carbon abundance is that an embryonic planet like Mercury, which had already formed a silicon-rich core, collided with and was absorbed by Earth," Dasgupta said. "Because it's a massive body, the dynamics could work in a way that the core of that planet would go directly to the core of our planet, and the carbon-rich mantle would mix with Earth's mantle.
"In this paper, we focused on carbon and sulfur," he said. "Much more work will need to be done to reconcile all of the volatile elements, but at least in terms of the carbon-sulfur abundances and the carbon-sulfur ratio, we find this scenario could explain Earth's present carbon and sulfur budgets." (Space.com)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

