
Magnesium is considered to be a promising anode candidate for pairing with the sulfur cathode. Unlike the Li anode, Mg can be safely used as a metal anode without dendrite formation. Furthermore, Mg is chemically inert, abundant in the earth’s crust, and has a high theoretical volumetric capacity of 3832 mA h cm-1 because of its bivalent nature, significantly higher than 2062 mA h cm-1 of lithium. However, the research of magnesium/sulfur (Mg/S) batteries is still in a very early stage. To date, a suitable electrolyte for Mg/S cells has yet to be reported, still urgently needs to be developed.
The research team led by Prof. ZHANG Yuegang from Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences (SINANO), proposed a simple method to synthesize a new type of mononuclear cation Mg salt ([Mg(THF)6][AlCl4]2) with high yield and high purity (Figure 1). They further employed this simple mononuclear salt to formulate a novel Mg electrolyte of [Mg(THF)6][AlCl4]2/PYR14TFSI/THF. The obtained Mg electrolyte possesses a highly reversible Mg plating/stripping cycling behavior (>100 cycles), good anodic stability (2.5 V vs. Mg) and much higher ionic conductivity (8.5 mS/cm) than most of the reported Mg electrolytes (Figure 2).
This new electrolyte with electroactive species of mononuclear Mg cation salt ([Mg(THF)6]2+) was used for a Mg/S cell and realized an initial discharge specific capacity about 700 mA h g-1. As shown in Figure 3, the cell can cycles stably for more than 20 cycles, which is better than the previous report using an electrochemically active cation ([Mg2(μ-Cl)3(THF)6]2+) based electrolyte (by Muldoon and co-workers in 2011). This work provides a new electrolyte with a more simple structure and better compatibility with Mg/S batteries, opens the door to the discovery of new magnesium salt based electrolytes and the fast development of Mg/S batteries.
The research results have been published in Angewandte Chemie International Edition, 2016, 55, 6406–6410. The work in SINANO was supported by the National Natural Science Foundation of China and Suzhou Science and Technology Development Program.

Figure 1 Proposed formation mechanism and X-ray crystal structure of Mg salt. (Image by SINANO)

Figure 2 Electrochemical performance of electrolyte ([Mg(THF)6][AlCl4]2) in PYR14TFSI/THF (1:1 v/v). (Image by SINANO)
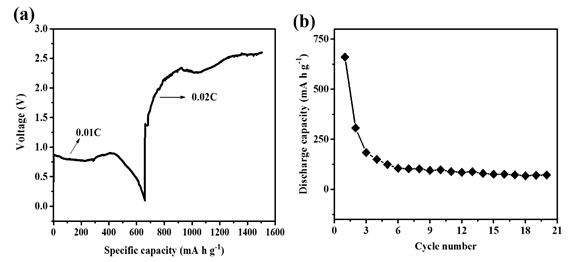
Figure 3 Discharge and charge profile for a) first cycle and b) 20 cycles of discharging and charging. Cathode: 50% S loading, NG/SP/Commercial S/PVDF=4:5:10:1 Rate: 0.01 C discharging, 0.02 C charging. Anode: Mg disk. (Image by SINANO)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

