
Haem is a ubiquitous prosthetic cofactor of proteins in aerobic organisms, such as myosin, hemoglobin, cytochrome c, catalase, peroxidase and so on. Under some pathological conditions, severe hemolysis occurred, which leads to a significant increase of free haem. Due to the capability of performing redox reactions, free haem can generate reactive oxygen species (ROS), causing oxidative stress and imposing toxic effects on tissues/organs, and cellular macro-biomolecules such as lipids, DNA, and proteins.
Dityrosine cross-linking is an important form of oxidative damage by heam. It has been reported that several disease-associated proteins can be aggregated via dityrosine cross-linking induced by heam in the presence of hydrogen peroxide (H2O2). However, it is still unclear that why do different proteins show different aggregation efficacy and whether or not the structural property of proteins plays the critical role.
To cope with this, a study team led by Prof. HUANG Qing in Institute of Technical Biology & Agriculture Engineering, Hefei Institutes of Physical Science, explored the effect of protein structure and/or conformation on the dityrosine cross-linking induced by haem-H2O2.
The researchers selected bovine serum albumin (BSA, globular protein), fibrinogen (fibrous protein), and beta-casein (natively disordered protein) as the protein samples and they found that protein structure has appreciable impact on the aggregation efficacy.
The intrinsically disordered and denatured protein are the most susceptible one to be aggregated, followed by the fibrous protein while the globular protein is hardly to be aggregated.
Based on the Raman and electron-spin-resonance (ESR) spectroscopy measurements, the researchers found that the protein structure affects not only the coordination state (five or six-coordinated) of protein with haem, but also the formation of tyrosyl radicals (the precursor of dityrosine). In fact, the 3D-structure of protein determines the susceptibility of the reaction of two tyrosyl radicals, and consequently the protein aggregation via dityrosine cross-linking.
This research was published in Biochimica et Biophysica Acta (BBA)-General Subjects entitled “Effect of protein structure and/or conformation on the dityrosine cross-linking induced by haem-hydrogen peroxide”.
This work was supported by the National Nature Science Foundation of China, the Youth Innovation Promotion Association of the Chinese Academy of Sciences, and also by the Hundred Talent Program of the Chinese Academy of Sciences when initiating this study in the beginning. And this study was conducted also with the help of High of Magnetic Field Laboratory of the Chinese Academy of Sciences in the ESR measurements.
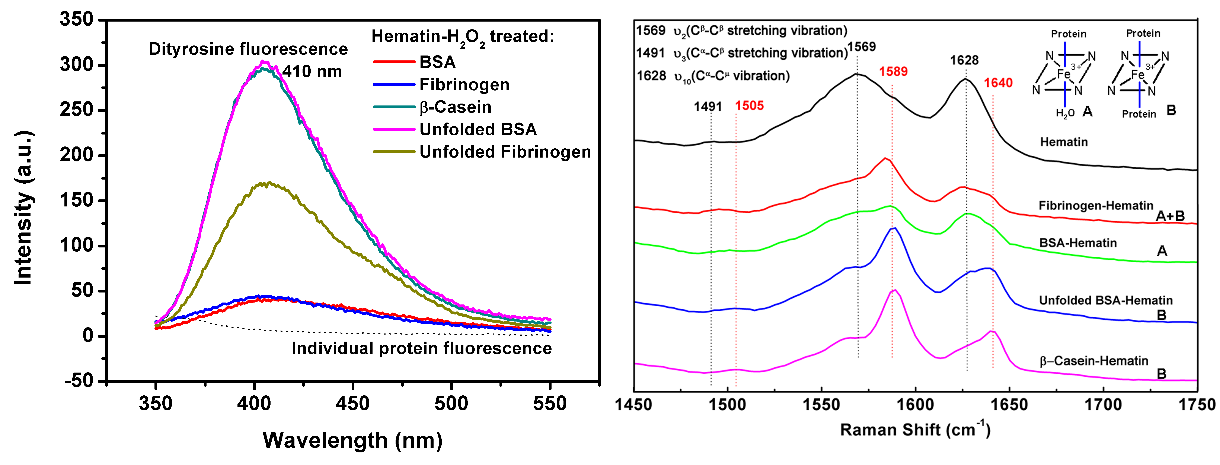
Left: Characterization of dityrosine cross-linking of different protein by haem-H2O2 with fluorescence spectroscopy; Right: Characterization of the coordination state of different protein with haem with Raman spectroscopy. (Imaged by KE Zhigang)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

