
Since the Fukushima accident in 2011, the possibility of harm to ecological environment and human health caused by radioactive pollution has aroused wide public concern. Uranium is likely to be released into the environment during the mining, processing and transport process, resulting in radioactive pollution. It is important to understand how uranium is transported and transformed on earth surface in order to assess its impact on environment and human health.
In the past, researches generally focused on the effect of different environmental conditions on U(VI) at water-mineral interface absorption. However, studies concerning the effect of microbes on the adsorption of radionuclides at water-mineral interface were rarely reported.
Dr. SUN Yubing and his co-workers, researchers in Institute of Plasma Physics, Hefei Institutes of Physical Science, has been investigating the environmental factors, i.e., reaction time, pH, ionic strength and initial concentration, on the physicochemical behaviors and micro-structures of radionuclides at water-solid interfaces by using batch, synchrotron X-ray absorption spectroscopy (XAS) and surface complexation modeling, to achieve better understanding of Transports of Radionuclides at Water-mineral-microbe Ternary Systems. This time, they studied the interaction mechanism between U(VI) and sericite in the presence of microbes at the molecular level by batch adsorption, synchrotron radiation technique (EXAFS) and surface complexation modeling.
They found that microbes (Bacillus subtilis) facilitated the adsorption of U(VI) on sericite at pH < 5.0, whereas the decreased adsorption of U(VI) was observed at pH > 6.0 due to the strong complexation of deprotonated carboxyl groups of Bacillus subtilis and sericite.
According to EXAFS analysis, the inner-sphere surface complexation of U(VI) on sericite+B. subtilis at pH 7.0 was demonstrated due to the formation of U-Si/Al (0.38 nm ) and U-C shell (0.292 nm), whereas the U-P shell (0.365 nm)) was observed at pH 4.0. The effect of B. subtilis on U(VI) adsorption onto sericite can be satisfactorily fitted by surface complexation modeling using ion exchange and inner-sphere surface complexation (i.e., phosphoryl, carboxyl and hydroxyl groups) under the range of allowable error.
These observations indicated that microbes significantly influenced the U(VI) adsorption on the clay mineral under various geological conditions.
Dr. SUN’s observations play an important role in understanding the fate and transports of radionuclides at water-mineral-microbe ternary systems. And their study was published in Geochimica et Cosmochimica Acta with title Adsorption of U(VI) on sericite in the presence of Bacillus subtilis: A combined batch, EXAFS and modeling techniques.
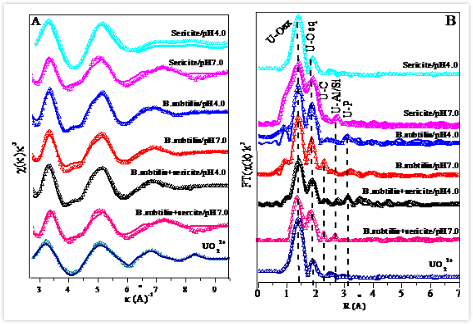
EXAFS spectra (A) and their corresponding Fourier transform (FT) (B) for U(VI)-containing sericite, B. subtilis and sericite+B.subtilis. (Image by SUN Yubing)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

