
Chronic myeloid leukemia (CML), a hematological cancer of bone marrow white blood cells, constitutes about 15% of adult leukemia and usually 1-2 patient is diagnosed with CML per 100,000 people/per year in US. It is characterized by a reciprocal chromosomal translocation between chromosome 9 and 22 of the break point cluster region (BCR) gene with the Abelson (ABL) gene for ABL kinase, which leads to a shortened chromosome 22. The fusion tyrosine kinase BCR-ABL is constitutively active and causes uncontrolled myeloid cell proliferation through downstream mediators such as Stat5 and extracellular signal-regulated kinase (ERK).
Despite of the great clinical success, the Food and Drug Administration (FDA) approved that targeting BCR-ABL kinase for the CML drugs all potently inhibit other targets such as DDR1/2, c-KIT and so on besides ABL kinase. Although the role of off-targets inhibition in the clinical aspect remains unclear, the highly selective BCR-ABL inhibitor is still highly demanded as far as both the preclinical pathological and clinical side effects mechanistic study concerned.
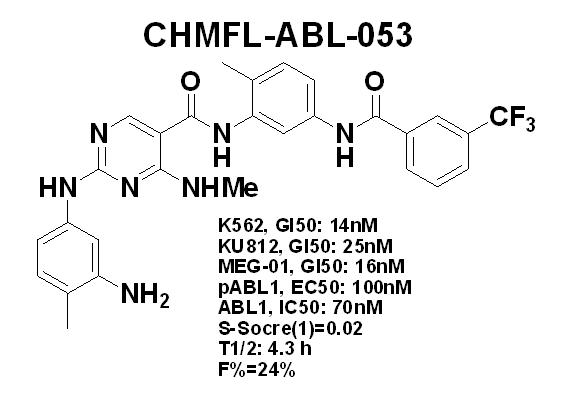
Based on the X-ray structure of ABL kinase, a study team led by LIU Qingsong and LIU Jing from High Magnetic Field Laboratory,Hefei Institutes of Physical Science, Chinese Academy of Sciences (CHMFL) designed a highly potent (ABL1: IC50 of 70 nM) and selective (S score (1) = 0.02) BCR-ABL kinase inhibitor, CHMFL-ABL-053. The CHMFL-ABL-053 did not exhibit apparent inhibitory activity against c-KIT kinase, which is the common target of currently clinically used BCR-ABL inhibitors. CHMFL-ABL-053 almost completely suppressed BCR-ABL kinase autophosphorylation at the Y245 site in K562, KU812 and MEG-01 at the concentration of 300 nM. BCR-ABL kinase downstream mediator Stat5, crk-like protein (CrkL), and ERK phosphorylation was also significantly inhibited in a concentration-dependent manner.
In addition, even in the early 12 hours, CHMFL-ABL-053 could dose-dependently arrest the cell cycle progression in the G0/G1 phase in these cells. Upon 24 or 48 hours of drug treatment, 100 nM concentration of CHMFL-ABL-053 could significantly induce apoptotic cell death. CHMFL-ABL-053 displayed potent antiproliferation efficacy against K562 cells (GI50: 0.014 μM) and P210-BaF3 cells (GI50: 0.007 μM) and exhibited good selectivity over parental BaF3 cells (GI50: > 10 μM). CHMFL-ABL-053 inhibited the proliferation of CML cell lines K562 (GI50 = 14 nM), KU812 (GI50 = 25 nM) and MEG-01 (GI50 = 16 nM), but not other AML cell lines, implying strong and selective on-target effects. Furthermore, CHMFL-ABL-053 did not display any apparent activity against the Chinese hamster lung (CHL) cell line, indicating a good nonspecific toxicity profile.
In the study of PK profiling in rats, compound CHMFL-ABL-053 exhibited good systemic exposure (AUC = 1715.51 ng/mL·h, Cmax = 367.61 ng/mL), favorable oral bioavailability (F = 24.19%), and acceptable half-life (t1/2 = 4.33 h) following oral administration of a single dose of 10 mg/kg. The in vivo antitumor study of CHMFL-ABL-053 was performed in the K562 cell inoculated xenograft mouse model. After 16 days of continuous treatment, compound CHMFL-ABL-053 dose-dependently inhibited the growth of the K562 tumor, and a 50 mg/kg/ day dosage could almost completely suppress tumor progression. As a potential useful drug candidate for CML, CHMFL-ABL-053 is under extensive preclinical safety evaluation now.
The results were published in ACS Journal of Medicinal Chemistry, entitled "Discovery of 2-((3-amino-4-methylphenyl)amino)-N-(2-methyl-5-(3-(trifluoromethyl)benzamido)phenyl)-4-(methylamino)pyrimidine-5-carboxamide (CHMFL-ABL-053) as a potent, selective and orally available BCR-ABL/SRC/p38 kinase inhibitor for Chronic Myeloid Leukemia”.
CHMFL-ABL-053 anti-tumor efficacy in K562 xenograft model. (Image by LIANG Xiaofei and ZOU Fengming)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

