
Scientists recently reported the relevance, significance and pathological impact of the Wnt pathway-associated factor SFRP2 on human health. For years, scientists persistently believe SFRP2 is a Wnt16 antagonist, as it tends to be genetically lost, hyper-methylated or downregulated in many solid tumors. However, the current new study revealed that SFRP2 in deed acts as a typical Wnt axis agonist by promoting WNT16B activities in the tumor microenvironment (TME) therapeutically damaged by anticancer treatments.
Tumors usually develop in complex tissue environments, which they depend on for sustained growth, invasion and metastasis. Unlike cancer cells, stromal cell types within the TME are genetically stable and thus represent an attractive therapeutic target with reduced risk of resistance and tumor recurrence. Specifically disrupting the pro-tumorigenic TME is a challenging issue, as the TME has diverse capacities to induce both beneficial and adverse consequences for tumorigenesis.
Investigators in the group of Dr. SUN Yu, Institute of Health Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences/Shanghai Jiao Tong University School of Medicine, recently found the paradoxical roles of the SFRP2 in the TME during specific stages of cancer progression under therapeutic conditions, as well as potential strategies to manipulate the TME by targeting a specific factor to improve clinical outcome in the settings of precision medicine.
They first noticed the expression of SFRP2 induced in stromal cell populations by genotoxic agents administered to prostate cancer patients, a phenomenon also observed in multiple cancer types including breast cancer and colorectal cancer populations. Further, SFRP2 upregulation is significantly and reversely correlated with overall survival of patients who received chemotherapy. Experimental data indicated that the transcription complex NF-kB is actively involved in SFRP2 overexpression. Co-immunoprecipitation assay proved that SFRP2 physically interacts with WNT16B in the TME once released from the stromal cells that are exposed to DNA damaging pressure.
Interestingly, functional activities of WNT16B were mostly enhanced by the stroma-derived SFRP2, although SFRP2 does not appear to have remarkable influences on cancer phenotypes. With a neutralizing monoclonal antibody against WNT16B, the malignant behaviors of prostate cancer cells were substantially abrogated in vitro, and diminished in vivo. Co-treatment of experimental mice xenografted with prostate cancer cells and stromal cells with chemotherapeutic agents and anti-WNT16B effectively reduced the average volume of tumors, by minimizing the side effects of genotoxic treatments.
Overall, the study opens a new avenue for rational development of combinational regimens for advanced tumors especially those with acquired resistance in the course of anticancer therapies. It provides a principal of proof for the efficacy of targeting strategies integrating monoclonal antibodies to deplete a single soluble factor from the treatment-remodeled TME.
The study titled “SFRP2 augments WNT16B signaling to promote therapeutic resistance in the damaged tumor microenvironment” was published online ahead of print in the journal Oncogene on January 11, 2016.
This project was financially supported by US DoD PCRP-Ideal Development Award, National Natural Science Foundation of China and Research Funding of Chinese Academy of Sciences.
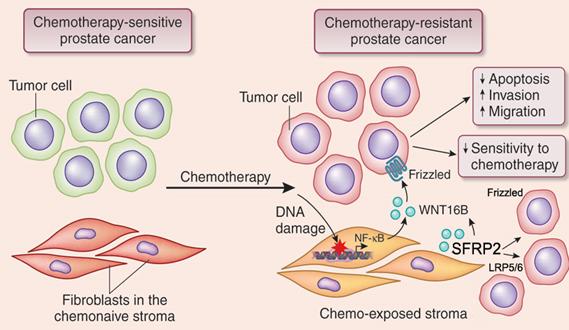
Figure legend: Upon DNA damage pressure imposed by chemotherapy or radiation in clinics, normal cell subpopulations in the tumor-adjacent stroma display a highly distinct, robust and chronic secretory phenotype. SFRP2, a soluble factor released from damaged stromal cells, can significantly consolidate the canonical Wnt pathway activated by WNT16B, another protein concurrently generated by the tumor microenvironment. Importantly, targeting WNT16B via monoclonal antibody in synergy with standard chemotherapy, remarkably abrogates cancer resistance acquired from the stroma, postpones tumor growth and improve preclinical outcome. This study provides valuable clue for future efforts in cancer resistance investigation and novel drug development. (Image by Prof. SUN Yu’s group)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

