
Obesity and its comorbidities, such as type 2 diabetes and insulin resistance, have become a major threat to human health globally in the 21st century. Obesity is associated with chronic inflammation, characterized by accumulation of adipose tissue macrophages (ATMs). These cells are pro-inflammatory macrophages (classically activated macrophages) that could promote metabolic disorders. Macrophages in lean adipose tissue display an alternatively activated phenotype, with the ability to guard insulin resistance. However, the mechanisms underlining macrophage accumulation in adipose tissue and the function of different macrophage types during obesity-related insulin resistance remain unclear.
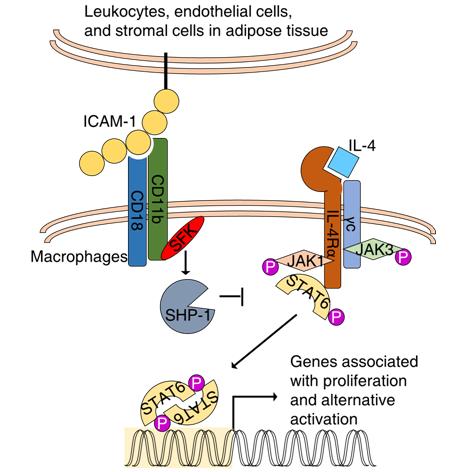
A team led by Drs. SHI Yufang and WANG Ying from the Institute of Health Sciences, Shanghai Institutes for Biological Sciences under Chinese Academy of Sciences just reported that CD11b (integrin αM) is important in mediating monocyte immigration into adipose tissue. In absence of CD11b, an enhanced in situ proliferation as well as the increased alternative activation of ATMs were observed, accompanied by reduced insulin resistance.
They found that CD11b negatively regulates IL-4/STAT6-induced proliferation of ATMs and polarization of the alternatively activated phenotype. This effect of CD11b depends on a complex signaling process involving members of the src family kinases and SHP-1.
This work was published online in PNAS on Dec. 15, 2015, as a research article entitled "CD11b Regulates Obesity-Induced Insulin Resistance via Limiting Alternative Activation and Proliferation of Adipose Tissue Macrophages".
This study was funded by the Scientific Innovation Project of the Chinese Academy of Science, the Ministry of Science and Technology of China, the Programs of National Natural Science of China, and etc.

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

