
Clostridium cellulolyticum and other cellulolytic clostridium strains can degrade lignocellulose and produce lignocellulosic biofuels and chemicals. The Metabolomics Group of Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) is working towards the construction of clostridial genetic manipulation platform with high efficiency and feasibility. On the basis of our previously developed genetic tools (J Microbiol Methods, 2012, 89, 201-208.; PLoS One 2013, 8, e69032; Appl Microbiol Biotechnol, 2014, 98, 313-323.), a novel arabinose-inducible genetic operation system has been developed recently, which will greatly promote the metabolic engineering for Clostridium and the industrial utilization of lignocellulosic feedstock. The results have been published in Biotechnology for Biofuels.
C. cellulolyticum and other cellulolytic strains can degrade cellulose effectively and produce cellulosic ethanol in one step. Thus, the industrial utilization of lignocellulose via the metabolic engineering of the clostridial strains is of great importance in solving the energy crisis and environmental problem in world-wide range. By far, although several genetic tools have been developed for Clostridium strains, including gene disruption methods via either homologous recombination or intron retrohoming mechanism and heterologous gene expression methods using replicative or integrative plasmids, inducible gene expression tools with high activity and stringency are still required.
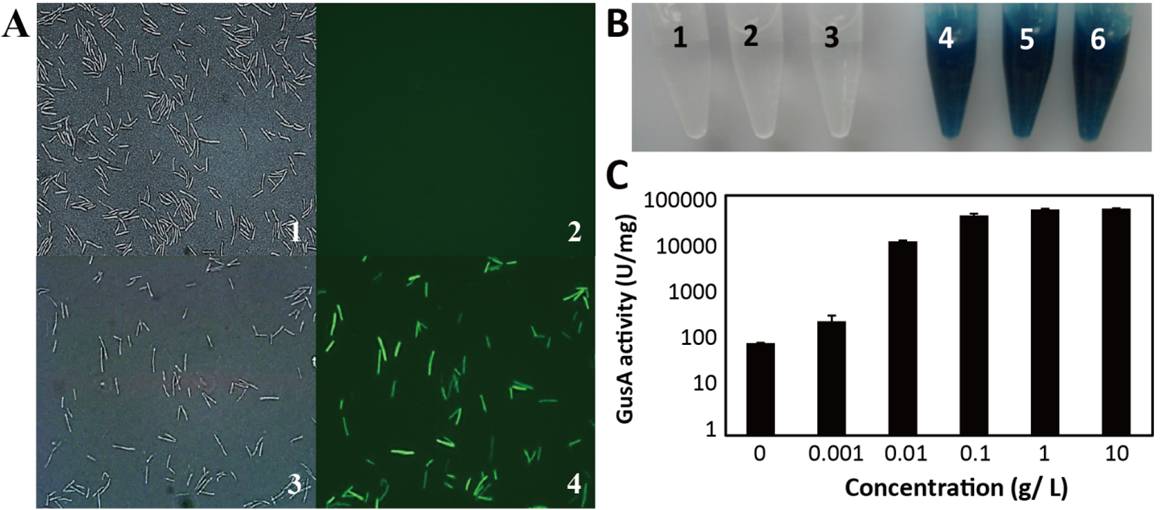
In this study, the researchers constructed a highly efficient arabinose-inducible gene operation system ARAi. This system includes an effective gene expression platform with an oxygen-independent fluorescent reporter (Figure 1A), a sensitive MazF-based counterselection genetic marker, and a precise gene knock-out method based on an inducible ClosTron system. The arabinose-inducible promoter in ARAi system is derived from Clostridium acetobutylicum. In the presence of the specific inducer L-arabinose, the gene expression level could be up-regulated over 800-fold (Figure 1B), which is the highest inducing activity for Clostridium according to the known knowledge. In addition, the inducible ClosTron method of the ARAi system decreases the off-target frequency from 100% to 0, which shows the precise gene targeting in C. cellulolyticum.
The arabinose-inducible gene operation system ARAi developed in this study has high inducing efficiency and stringency, and ARAi can be applied in both controllable gene expression and precise gene disruption. Therefore, this study provides a useful genetic tool for the systems biology and metabolic engineering of Clostridium, which will stimulate the application of Clostridium in the bioconversion of lignocellulosic substrates.
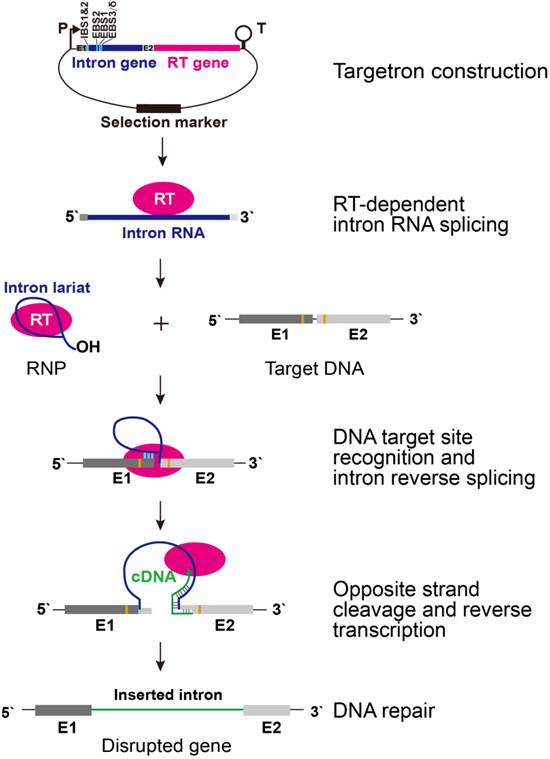
Based on the obtained results in the studies of mesophilic and thermophilic Clostridium strains, the Metabolomics Group of QIBEBT has published a review article in Biotechnology Journal, in which the mobile group II intron-derived targetron technology and its application in metabolic engineering have been summarized and illustrated (Figure 2). This review demonstrates the resent process of the Metabolomics Group in the field of genetic engineering for clostridial strains.
The studies on Clostridium strains in Metabolomics Group is supported by the National Basic Research Program of China (973 Program), the National Natural Science Foundation of China, and the Qingdao Science and Technology Development Plan.
 |
|
Figure 1. The inducing activity of ARAi system inC. cellulolyticum.A, Confirmation of the expression of oxygen-independent fluorescent protein byin vivofluorescent imaging. 1, 3 corresponds to cell morphology; 2, 4 corresponds to fluorescent imaging; 1, 2 shows cells without induction; 3, 4 shows cells with induction. B, Determination of inducible expression of GusA with X-gluc as a substrate. The blue colors of the reaction solutions indicate the induced expression of GusA. The numbered tubes contained: 1-3, negative control without crude enzyme or the inducer L-arabinose; 4-6, with 0.1, 1 and 10 g/L L-arabinose, respectively. C, Determination of the inducible expression of GusA using MUG as a substrate. In the presence of the inducer, the gene expression level could be up-regulated over 800-fold. (Image by QIBEBT) |
 |
|
Figure 2. Targetron structure and retrohoming mechanism for targeted gene disruption. P, promoter; T, transcription terminator; RT, reverse transcriptase; RNP, rebonucleoprotein; E1, exon at 5’ of target DNA sequence; E2, exon at 3’ of target DNA sequence. Intron RNA and DNA target site base-pairing interaction is indicated by light blue bars. Orange highlights indicate important base-pairs in E1 and E2 of target DNA recognized by RT. (Image by QIBEBT) |
References:
A novel arabinose-inducible genetic operation system developed forClostridium cellulolyticum, Biotechnology for Biofuels, 2015, 8, 36.
Current progress of targetron technology: Development, improvement and application in metabolic engineering, Biotechnology Journal, 2015, 10, 855.
Contacts:
Prof. CUI Qiu; Dr. LIU Yajun
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
E-mail: cuiqiu (AT) qibebt.ac.cn; liuyj (AT) qibebt.ac.cn

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

