
Coronary artery disease causes myocardial infarction, the leading cause of death worldwide. How coronary arteries develop is a fundamental biological question with important ramifications for human health and disease. Defining the developmental programs that give rise to the coronary arteries will provide critical information for regenerative approaches to congenital and adult heart diseases. Most previous studies of coronary developmental origins have focused on the midgestation stage, when coronary vessels initially form over the heart. Postnatal coronary vessels were presumed to arise from these embryonic coronary vessels, but few studies have examined postnatal coronary artery growth, and this assumption has not been rigorously tested.
To address this assumption, ZHOU Bin, professor of the Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences and his group members performed genetic lineage tracing of embryonic coronary vessels. They discovered that a substantial portion of postnatal coronary vessels arise de novo in the neonatal mouse heart, rather than expanding from preexisting embryonic vasculature.
They designated two distinct coronary vascular populations (CVPs) that arise through different developmental mechanisms and that are spatially segregated in their location. The 1st CVP starts from sub-epicardial precursors, which generate the initial coronary vascular plexus of the fetal heart and which ultimately gives rise to the vessels of the outer myocardial wall of the neonatal heart. The 2nd CVP derives from ventricular endocardial cells and forms the coronary vasculature of the inner myocardial wall during the postnatal period and of the ventricular septum.
At the same time, they observed that neonatal endocardial cells transform from a single sheet lining the heart lumen into many tube-shaped blood vessels. The reservoir contains coronary vessel precursors in the form of endocardial cells. Lineage conversion of neonatal endocardial cells during trabecular compaction generates a distinct compartment of the coronary circulation located within the inner half of the ventricular wall. This lineage conversion occurs within a brief period after birth and provides an efficient means of rapidly augmenting the coronary vasculature.
These findings uncovered new origin and developmental mechanisms of mammalian coronary artery. This mechanism of postnatal coronary vascular growth provides avenues for understanding and stimulating cardiovascular regeneration following injury and disease.
This research entitled “De novo formation of a distinct coronary vascular populationin neonatal heart” was published in Science on July 4, 2014.
This study was supported by funds from Chinese Academy of Sciences, National Basic Research Program of China, National Natural Science Foundation of China, and Science and Technology Commission of Shanghai Municipality.
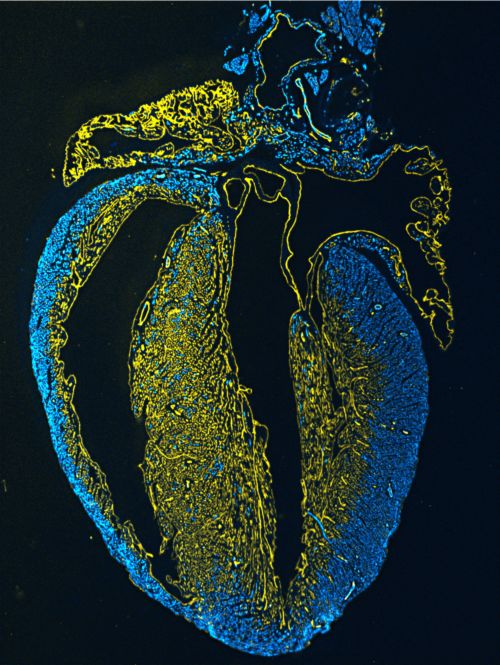
Two distinct coronary vascular populations (CVPs) visualized by lineage tracing.1st CVP (in blue) and 2nd CVP (in yellow). (Image by ZHOU Bin's research group)
CONTACT:
ZHOU Bin, Professor
Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences
Shanghai 200031, China
Tel: 86-21-54920974;
Fax: 86-21-54920291;
E-mail: zhoubin@sibs.ac.cn

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

