
Alkynylated arenes represent a recurring structural motif and useful synthetic intermediate found in bioactive natural products, pharmaceuticals and organic materials. The recent developed C-H bond functionalization reactions directly utilize carbon-hydrogen bond in place of carbon-halogen bond to circumvent the halogenation process of arenes, providing more efficient and concise synthetic routes to the target molecules. Consequently, the development of direct dehydrogenative cross-coupling reaction to synthesize alkynylated arenes is highly desirable.
The research group headed by Prof. SU Weiping at Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, has reported the first direct alkynylation of thiophenes with terminal alkynes.
This direct alkynylation method developed by Prof. SU’s group employs low loading of palladium salt as catalyst and silver salt as oxidant to suppress the undesired homo-coupling of terminal alkynes. In addition to thiophenes, the protocol is also applicable to other electron-rich heterocycles and tolerates a broad range of functional groups, presenting a versatile method for the facile syntheses of alkynylated heterocycles.
In general, this is the first general method for the direct synthesis of alkynylated heterocycles using terminal alkynes. The strategy used to suppress alkyne homo-coupling would be useful for developing more efficient direct alkynylation reactions in the future.
Results of this study have been published as VIP (very important paper) in Angew. Chem. Int. Ed. (2013, 52, 3630-3633).
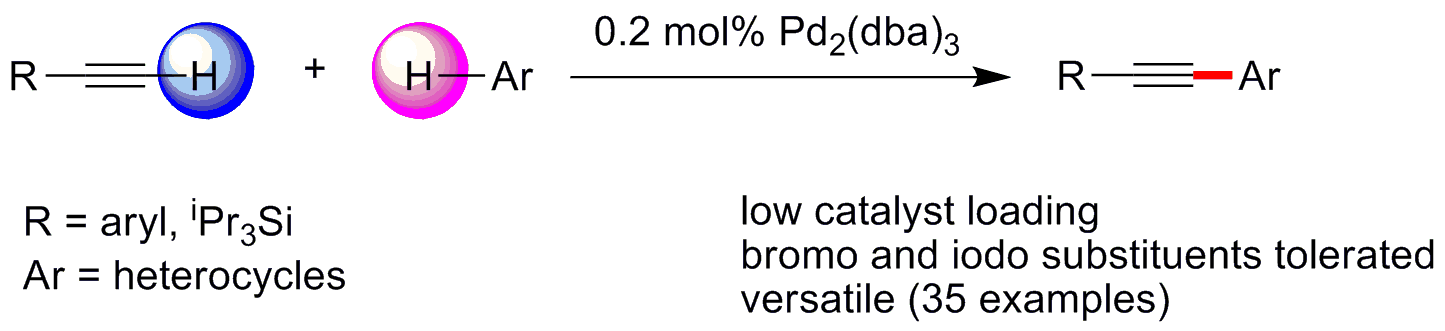
Schematic illustration of a general method for the direct alkynylation of heterocycles (Image by Prof. SU Weiping' s group)
CONTACT:
Prof. SU Weiping
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: wpsu@fjirsm.ac.cn

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

