
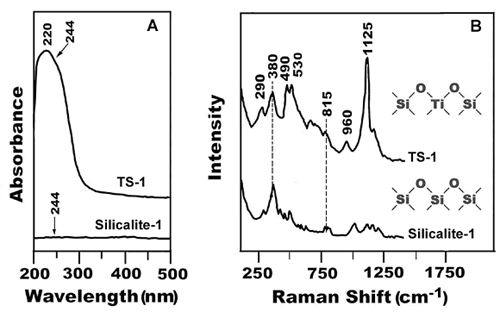
 UV-visible DRS and UV resonance Raman spectra of TS-1 and Silicalite-1 [J. Phys. Chem. B2003, 105: 2993; | |
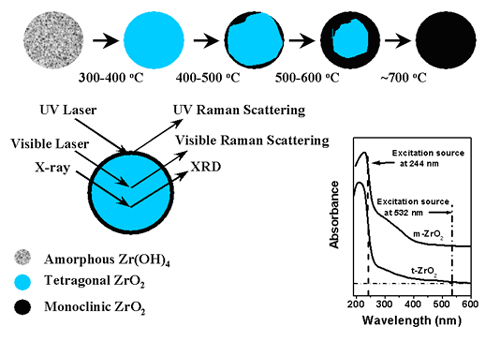 The information from UV and Visible Raman and XRD of ZrO2 calcined at different temperatures. The Insert is UV-vis DRS of monoclinic and tetragonal ZrO2. [J. Phys. Chem. B,2001,105:8107; |
(1) Identification of Isolated Transition Metal Ions in Molecular Sieves and on Oxide Supports
The isolated transition metal ions in the framework of molecular sieves (e.g.,TS-1, Fe-ZSM-5, Fe-SBA-15, Fe-MCM-41 and V-MCM-41) and their oxides on the surface of these sieves, and other oxides (e.g.,MoO3/Al2O3 and TiO2/SiO2) were successfully identified by UV resonance Raman spectroscopy. The local coordination of these ions in the rigid framework of molecular sieves or the relatively flexible structure on the surface can also be distinguished by the shifts of the characteristic resonance Raman bands. The content of the isolated transition metal ion/oxides could be estimated by the relative intensities of Raman bands. This study demonstrates that the UV resonance Raman spectroscopy is a general technique that can be widely applied to the in-situ characterization of catalyst synthesis and catalytic reactions
(2) Phase Transition of Oxide Materials Studied by UV Raman Spectroscopy
Characterization of the phase in the surface region of an oxide particle is a very interesting but challenging objective because many chemical and physical properties of metal oxides largely depend on the phase in the surface region. Our characterization results by UV Raman spectroscopy indicated that phase transformation of zirconia starts from its surface region and then gradually develops into its bulk. For Y2O3-ZrO2 and La2O3-ZrO2, the transformation of the bulk phase from the tetragonal to the monoclinic is significantly retarded. However the tetragonal phase in the surface region is difficult to stabilize, particularly when the stabilizers content is low. The phase in the surface region can be more effectively stabilized by lanthanum oxide than yttrium oxide even though zirconia seemed to provide more enrichment in the surface region of the La2O3-ZrO2 sample than the Y2O3-ZrO2sample, based on XPS analysis. The surface structural tension and enrichment of the ZrO2 component in the surface region of ZrO2-Y2O3 and ZrO2-La2O3 might be the reasons for the striking difference between the phase change in the surface region and bulk. Accordingly, the stabilized tetragonal surface region can significantly prevent the phase transition from developing into bulk when the stabilizers content is high.
We also studied the phase transformation in nanophase titania (TiO2), the different crystal phase on the surface and in the bulk during phase transformation of TiO2 are also found.
The phenomena that the crystal phase on the surface and in the bulk during phase transformation could be quite common not only for the zirconia and titania, but also for many other oxides.

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

