
Bacteria and Archaea regularly face the threat of invading foreign DNA, such as phages, plasmids, transposons and so on. To protect themselves from invading genetic elements, prokaryotes have evolved many strategies. One of the most widespread is the CRISPR (clustered regularly interspaced short palindromic repeats)-associated system, also known as the Cas system.
The CRISPR-Cas system processes adaptive immunity in three steps. Phase I is called spacer acquisition. In this stage, short foreign-derived sequences called “spacers” are selected and incorporated into a CRISPR array from the invading element by the core proteins Cas1 and Cas2. In phase II (expression), spacers are transcribed into a pre-crRNA and then processed into mature crRNA that binds Cas proteins and forms the ribonucleoprotein complex. In the last interference phase, crRNA guides the resulting surveillance complex to recognize and degrade invading DNA complementary to crRNA. In recent years, the molecular mechanisms of the expression and interference phases were well characterized, however, spacer acquisition was the least understood aspect.
Memory of the invading nucleic acid is the key feature of the CRISPR-Cas system. Subsequent invasion by the foreign element triggers the memory bank to defend against invaders by using “acquired information”. Therefore, studying the acquisition phase is of great significance to understanding the whole CRISPR-Cas system.
As part of an extensive study, WANG Jiuyu, LI Jiazhi and colleagues, under the leadership of Prof. WANG Yanli at the Institute of Biophysics of the Chinese Academy of Sciences, solved the crystal structures of Cas1-Cas2 with a series of DNA. This research showed that the spacer acquisition step is PAM-dependent and provides a significant structural basis for the adaptive immunity mechanism.
The crystal structures of Cas1-Cas2-DNA complexes reveal that two Cas1 dimers sandwich a Cas2 dimer to form a flat DNA binding surface. Only DNA in a dual-forked configuration can be selected to be a protospacer, and the 3’-overhangs of the prostospacer are essential for new spacer acquistion.
This study also highlights that each Cas1 dimer has one catalytic subunit, which recognizes the PAM sequence in order to select the correct protospacer, and cleaves the 3’-overhangs to generate two 3’-OH groups.
On the basis of discovering the exact DNA substrate used by Cas1-Cas2 in vivo, the team showed that the architecture of the Cas1-Cas2 complex determines the length of the newly acquired spacer, and the Cas1-Cas2 complex undergoes a large conformational change upon DNA binding, just like a butterfly dropping its wings from “wings up” to “wings down.”
Building on the detailed messages provided by the structures of the various Cas1-Cas2-DNA complexes, Cas1 and Cas2 reveal their specific roles in spacer acquisition and how they recognize the PAM complementary sequence via sequence-specific interactions, which sheds light on the molecular basis for the selection of the protospacer using PAM. Together, this structural research provides critical insights into the molecular mechanism of the adaptation process, which will significantly facilitate research on this adaptive immunity system and pave the way for better development and utilization of the CRISPR-Cas system.
This study, entitled “Structural and Mechanisitic Basis of PAM-Dependent Spacer Acquisition in CRISPR-Cas Systems”, was published online by Cell on Oct. 15, 2015.
The research was funded by the Natural Science Foundation of China (91440201), the Chinese Ministry of Science and Technology (2014CB910102) and the Strategic Priority Research program of the Chinese Academy of Sciences (XDB08010203). The X-ray diffraction data were collected from beamlines BL-17U, BL-18U, and BL-19U at the Shanghai Synchrotron Radiation Facility.
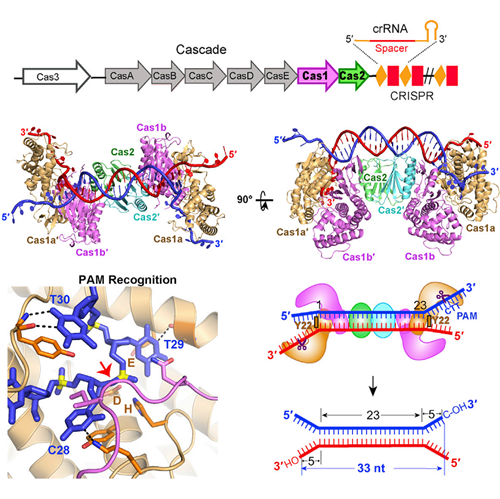
Crystal Structure of E. coli. Cas1-Cas2 bound to dual-forked protospacer DNA. Cas1-Cas2 recognizes and binds to dual-forked DNA containing a PAM-complementary sequence, then predetermines the protospacer length prior to inserting it into the CRISPR-Cas locus of E. coli. (© cell)
Contact:
WANG Yanli
Institute of Biophysics, Chinese Academy of Sciences
E-mail: ylwang@ibp.ac.cn

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

