
The neutralization of the car's tail gas is a problem of practical importance in the eyes of both experimental and theoretical physicists. Recently, a group of CAS scientists in Shenyang City join hands with The Queen's University of Belfast in the UK to make advances in exploring the process of CO oxidation in a bid to wipe out the air pollution caused by the car's exhaustive gas. The work has been supported by the "National 973 Program" and the CAS Foundation for Studying Abroad. On March 4, its result was published by the Internet edition of the Journal of the American Chemical Society.
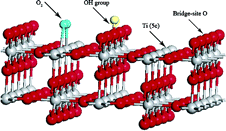
In the course of screening the workable catalysts, scientists discovered that a composite material composed of gold and oxides can trigger the catalytic reaction at an in-door temperature and water plays a special role in maintaining the reaction to go through until the reaction's end. But the origin of the oxygen atom and the critical role played by water in the CO oxidation at the interface between the gold and oxide are still two issues to be solved for demystifying the puzzling process.
The research group of computational materials science is an R&D body attached to the solid atomic imaging division under the Shenyang National Laboratory for Materials Science, a research team of the CAS Institute of Metal Research located in the capital of northeast China's Liaoning Province. Its researchers are engaged in the probing of a material's interface, its structural coupling, mechanical and physical properties.
Collaborated with Prof. HU Peijun at the Queen's University of Belfast, Dr. LIU Limin in the group first stood out to cope with the challenging issues by adopting the first-principles calculation to make clear water's role in the catalytic process to transform the poisonous gas CO into CO2 and the oxygen's diffusing process on the surface of TiO2.
The scientists reveal that the transfer of oxygen molecules and water's constructive role are closely related to one another. At first, the water is dissociated to OH and enables the TiO2 surface to absorb oxygen molecules meanwhile O2 is able to diffuse freely on the surface. So actually, water's critical role in the process is to transform the oxide to an inexhaustible reservoir in providing the oxygen molecules.

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

