
Opioid analgesics such as morphine are the most powerful and widely-used drugs to relieve pain in clinical treatment. They largely work through the μ-opioid receptors in the central nerous system, alleviating the perception of pain. But repeated application of the drugs within a certain period of time could lead to side-effects, like addiction and tolerance. In order to develop new effective painkillers with less side-effect, researchers strive to have a deeper understanding of the mechanism responsible for the analgesic efficacy of the drug and the formation of its adverse effects.
Through their studies on a neuropeptide called substance P, a group of CAS scientists have unveiled a new mechanism for the regulation of opioid analgesia, the process to reduce the effect of pain with opioid analgesics. Their work was reported in the August 25 issue of the prestigious journal
Cell.
Ever since it was first discovered by Swedish scientists as early as in the 1930s, substance P has been regarded as a main neurotransmitter of the pain signal in the spinal cord, and it is associated with the nervous system involved in the modulation of pain. However, the substance P antagonists, chemicals developed by pharmaceuticals to block the substance P receptor, have so far failed to relieve pain. This arouses a dispute over the role of the substance in the spinal cord.
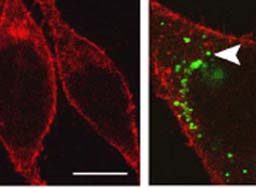
A research group led by Dr. Zhang Xu at the Institute of Neuroscience, the CAS Shanhgai Institutes for Biological Sciences, and their collaborators recently found the direct interactions between the newly-synthesized precursor of substance P and δ-opioid receptors, which co-exist and interact with the μ-opioid receptors in the primary sensory neurons. They also discovered that the substance P precursor could sort the δ-opioid receptors into regulated nerve pathways, allowing the surface insertion of the δ-opioid receptors in primary sensory neurons, either by stimuli or pain signals. Through integration with responsive stimuli, they can produce the pain-killing effects.
In addition, the CAS scientists succeeded in observing that the δ-opioid receptors could not be carried to the end of pain-transmission nerve fibers in a mouse's spinal cord when its genetic capability of producing the substance P has been deleted. It turns out that the mouse could not produce the effect of morphine tolerance. This fact proves that the substance P-regulated δ-opioid receptors play a critical role in regulating the formative process of morphine tolerance.
The research radically changes the traditional concepts and conventional understanding of the pain-regulating system composed of the substance P and opioids/opioid receptors. It provides a brand-new theoretical groundwork for developing new pain-killing medicines.






