
Newsroom
In a new study published on July 18 in Science Advances, a collaborative research team from the Institute of Biophysics of the Chinese Academy of Sciences (CAS), the Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou)/Institute of Microbiology of CAS, and the China University of Geosciences (Beijing), has unveiled unprecedented structural and mechanistic insights into Sulfolobus ellipsoid virus 1 (SEV1)—a hyperthermophilic archaeal virus thriving in one of Earth's most hostile environments.
SEV1, identified in a boiling acidic hot spring in Costa Rica, stands out for its ellipsoidal shape and lipid envelope-a rarity among archaeal viruses. Unlike more rigid viral structures, SEV1 exhibits remarkable flexibility while maintaining exceptional resilience at high temperatures and low pH levels.
Using cryo-electron tomography (cryo-ET), the researchers enriched SEV1 virions and and revealed a distinctive internal organization. Analysis revealed that the viral nucleoprotein filament winds multiple times within a plane along the longitudinal axis of the virus, forming disc-like structures resembling mosquito coils. These discs are stacked to generate a compact, spiral-shaped "spool" architecture.
Biochemical analyses identified VP4, encoded by the SEV1 genome, as the major capsid protein. Based on AlphaFold structural predictions and protein-DNA interaction modeling, VP4 was found to form dimers, with each monomer comprising five α-helices. Integrating structural and bioinformatic analyses, the researchers proposed a novel genome packaging mechanism mediated by VP4-termed the "coil-stacking" model.
Notably, the SEV1 virion remained structurally intact under high-temperature conditions. However, when stripped of its lipid envelope, the nucleocapsid structure became completely disrupted, indicating that the envelope plays a crucial role in stabilizing the viral genome under extreme environmental stress.
Subsequently, the researchers used cryo-focused ion beam (cryo-FIB) milling in combination with cryo-ET to visualize the infection cycle of SEV1 in its archaeal host. The results demonstrated that SEV1 assembly and envelope acquisition occur entirely within the host cytoplasm. Mature virions are released through virus-associated pyramids (VAPs), hexagonal pyramid-like structures that form on the host cell surface and open to allow progeny virus egress.
This study provides a detailed depiction of SEV1 assembly, envelopment, and release within host cells, and represents the first application of cryo-FIB in investigating the life cycle of an archaeal virus. It reveals the unique assembly and release strategies evolved by archaeal viruses to survive in extreme environments.
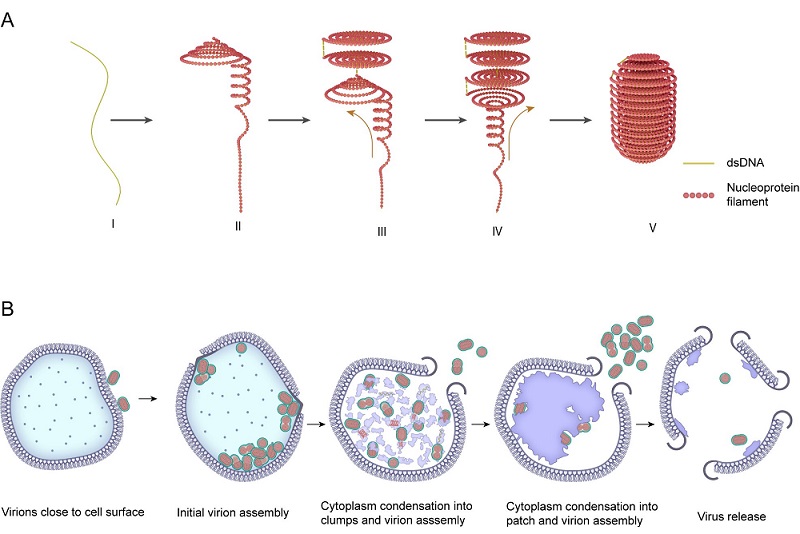
The genomic assembly and infection process of SEV1 (Image by ZHU Ping's group)
