Newsroom
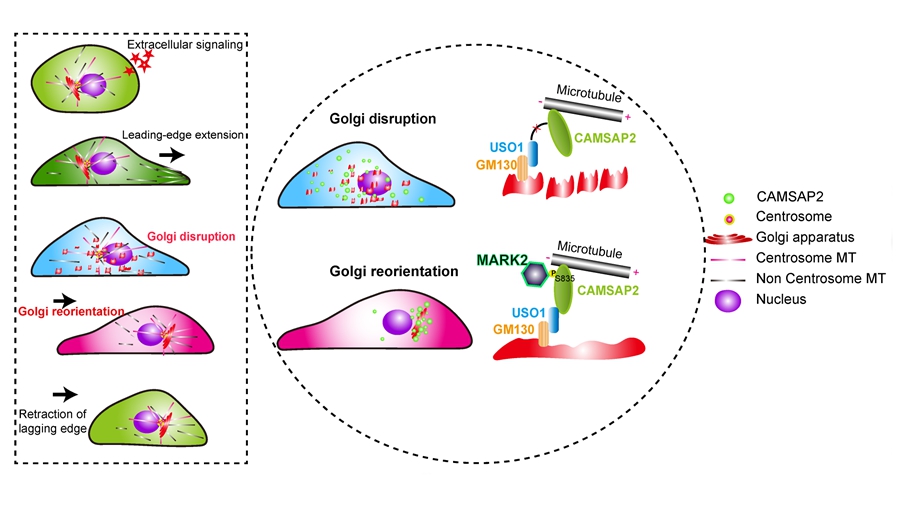
Cell migration is essential for various physiological processes, including embryonic development, tissue repair, and immune responses. A crucial aspect of directed cell migration involves the polarized trafficking of cellular components, with the Golgi apparatus playing a central role. The Golgi sorts newly synthesized proteins and membranes, directing them to specific regions of the cell, including the leading edge. Golgi-derived secretory cargo provides essential membrane materials, cell-surface receptors, and extracellular matrix components required for sustaining the forward movement of migrating cells. Despite its importance, the pathways that regulate Golgi reorientation during migration remain poorly understood.
In a study published in eLife on March 26, a team of researchers led by MENG Wenxiang at the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Sciences identified a key molecular pathway involved in this process. The research, for the first time, reveals how MARK2, a kinase critical for establishing cellular polarity, regulates the Golgi apparatus's reorientation by phosphorylating CAMSAP2, a protein that anchors non-centrosomal microtubules, in the reorientation of the Golgi apparatus during directed cell migration.
This study was supported by grants from the National Key R&D Program of China and the National Natural Science Foundation of China.