A mechanistic understanding of brain development requires a systematic survey of neural progenitor cell types, their lineage specification and maturation of postmitotic neurons. Cumulative evidences based on single-cell transcriptomic analysis have revealed the heterogeneity of cortical neural progenitors, their temporal patterning and the developmental trajectories of excitatory and inhibitory neurons in the developing neocortex. Nevertheless, the developmental hierarchy of the hypothalamus, which contains an astounding diversity of neurons that regulate endocrine, autonomic and behavioral functions, has not been well understood.
Recently, however, Prof. WU Qingfeng's group from the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences (CAS) conducted a study focusing on the origin of this neuronal diversity. For their work, they profiled the transcriptome of 43,261 hypothalamic neural cells to map the developmental landscape of the mouse hypothalamus and mapped the trajectory of radial glial cells (RGCs), intermediate progenitor cells (IPCs), nascent neurons and peptidergic neurons.
The researchers found that RGCs adopt a conserved strategy for multipotential differentiation, but generate both Ascl1+ and Neurog2+ IPCs. Ascl1+ IPCs differ from their telencephalic counterpart by displaying fate bifurcation whereby they can differentiate into both glutamatergic (excitatory) and GABAergic (inhibitory) neurons. Postmitotic nascent neurons derived from IPCs further resolve into multiple peptidergic neuronal subtypes. Clonal analysis also demonstrates that single RGCs can produce multiple neuronal subtypes.
This finding reveals that multiple cell types along the lineage hierarchy contribute to the fate diversification of hypothalamic neurons in a stepwise fashion, thus uncovering an effective strategy for neural progenitors to generate extreme neuronal diversity.
Furthermore, this study provides a developmental perspective for understanding hypothalamus plasticity and gaining valuable insights into hypothalamic diseases such as anorexia, narcolepsy and insomnia.
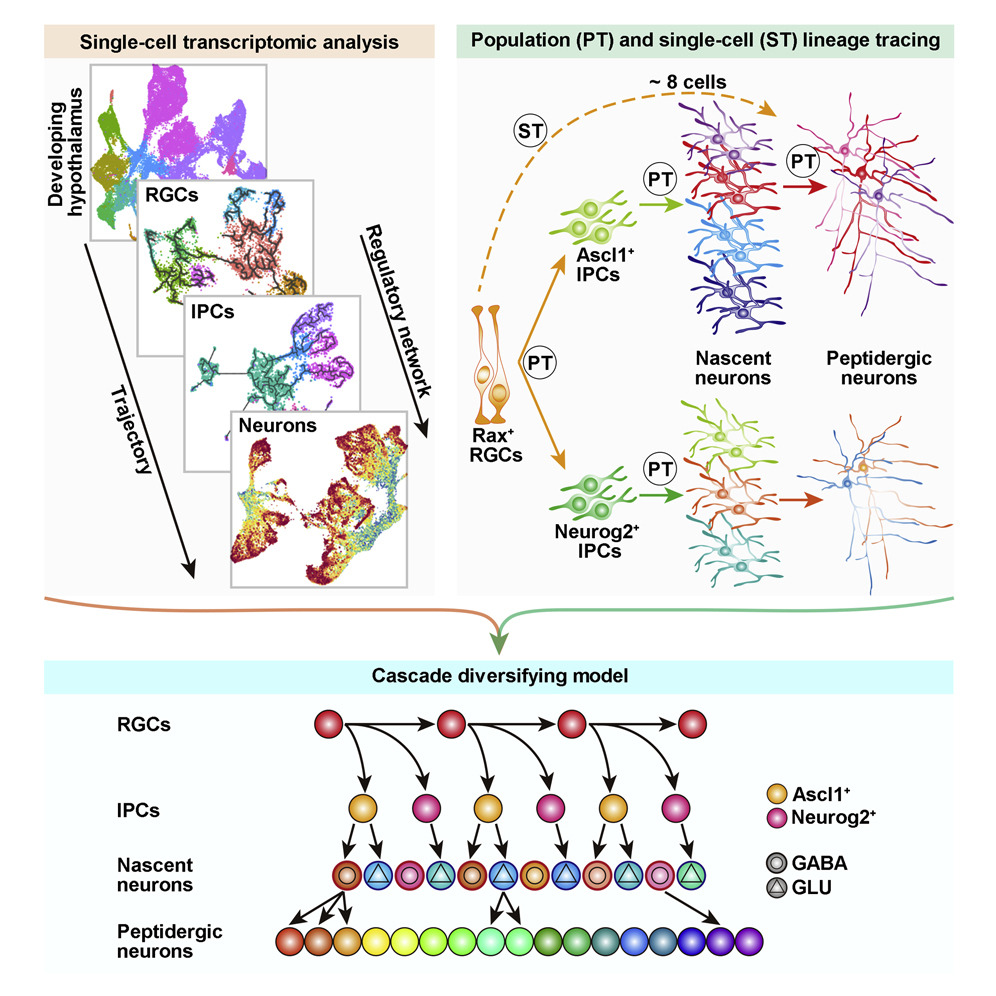
The cascade diversifying model for generating extreme neuronal diversity in hypothalamus (Image by IGDB)


