
Ferrate (VI) Oxidative Removes Various Organic Micropollutants
Oct 10, 2014 Email"> PrintText Size
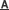
Emerging organic contaminants have been continually introduced to the aquatic environment and caused serious pollution of water sources which poses potential risks to human health. As a subsequent coagulant and precipitant, ferrate (VI) (Fe (VI)) has been widely used to remove organic pollutants, heavy metals, and pathogens. Nevertheless, there has been no intensively study on the removal of emerging organic contaminants by Fe (VI) available in the literature, especially on the reaction mechanisms.
Prof. YING Guangguo’s research group from Guangzhou Institute of Geochemistry of the Chinese Academy of Sciences (GIGCAS) evaluated the potential of Fe (VI) oxidation of the brominated flame retardant tetrabromobisphenol A (TBBPA) by using reaction kinetics, products identification and toxicity evaluation. They investigated the influencing effects of humic acid and clay particles on Fe (VI) removal of TBBPA in comparison with bisphenol A (BPA). The obtained apparent second-order rate constants (kapp) for Fe (VI) reaction with TBBPA ranged from 7.9(±0.3)×103 M−1 s−1 to 3.3(±0.1)×101 M−1 s−1 with the half-life (t1/2) ranging from 1.7 s to 419.3 s at pH 7.0-10 for an Fe(VI) concentration of 10 mg L-1.
Easier oxidation by Fe (VI) was observed for TBBPA than for BPA. Fe (VI) can destroy and transform the TBBPA molecule through β-scission reaction and yieldi the chemical species of low bromine-substituted products. More importantly, the oxidation of TBBPA by Fe (VI) led to the loss of its multiple hormonal activities (androgenic, antiestrogenic and antiandrogenic activities). The organic component humic acid decreased the TBBPA and BPA reactions with Fe (VI), while the inorganic component montmorillonite had no effect on their removal within the tested concentrations. Increasing the Fe (VI) dosage can reduce the effects of soluble organic matter and clay particles present in source waters on the degradation process and lead to the complete removal of target micropollutants.
The results of this study clearly provide the scientific basis for the removal of selected emerging organic contaminants by Fe (VI) oxidation process. In conclusion, Fe (VI) oxidation technology appears to be a promising tool for applications of water and wastewater treatment and other decontamination processes.
This work has been published in Water Research.
Image by YING's research group of GIGCAS
Emerging organic contaminants have been continually introduced to the aquatic environment and caused serious pollution of water sources which poses potential risks to human health. As a subsequent coagulant and precipitant, ferrate (VI) (Fe (VI)) has been widely used to remove organic pollutants, heavy metals, and pathogens. Nevertheless, there has been no intensively study on the removal of emerging organic contaminants by Fe (VI) available in the literature, especially on the reaction mechanisms.
Prof. YING Guangguo’s research group from Guangzhou Institute of Geochemistry of the Chinese Academy of Sciences (GIGCAS) evaluated the potential of Fe (VI) oxidation of the brominated flame retardant tetrabromobisphenol A (TBBPA) by using reaction kinetics, products identification and toxicity evaluation. They investigated the influencing effects of humic acid and clay particles on Fe (VI) removal of TBBPA in comparison with bisphenol A (BPA). The obtained apparent second-order rate constants (kapp) for Fe (VI) reaction with TBBPA ranged from 7.9(±0.3)×103 M−1 s−1 to 3.3(±0.1)×101 M−1 s−1 with the half-life (t1/2) ranging from 1.7 s to 419.3 s at pH 7.0-10 for an Fe(VI) concentration of 10 mg L-1.
Easier oxidation by Fe (VI) was observed for TBBPA than for BPA. Fe (VI) can destroy and transform the TBBPA molecule through β-scission reaction and yieldi the chemical species of low bromine-substituted products. More importantly, the oxidation of TBBPA by Fe (VI) led to the loss of its multiple hormonal activities (androgenic, antiestrogenic and antiandrogenic activities). The organic component humic acid decreased the TBBPA and BPA reactions with Fe (VI), while the inorganic component montmorillonite had no effect on their removal within the tested concentrations. Increasing the Fe (VI) dosage can reduce the effects of soluble organic matter and clay particles present in source waters on the degradation process and lead to the complete removal of target micropollutants.
The results of this study clearly provide the scientific basis for the removal of selected emerging organic contaminants by Fe (VI) oxidation process. In conclusion, Fe (VI) oxidation technology appears to be a promising tool for applications of water and wastewater treatment and other decontamination processes.
This work has been published in Water Research.

Image by YING's research group of GIGCAS
CAS Institutes
There are 124 Institutions directly under the CAS by the end of 2012, with 104 research institutes, five universities & supporting organizations, 12 management organizations that consist of the headquarters and branches, and three other units. Moreover, there are 25 legal entities affiliated and 22 CAS invested holding enterprisesThere are 124 I...>> more
Contact Us

Chinese Academy of Sciences
Add: 52 Sanlihe Rd., Xicheng District, Beijing, China
Postcode: 100864
Tel: 86-10-68597592 (day) 86-10-68597289 (night)
Fax: 86-10-68511095 (day) 86-10-68512458 (night)
E-mail: cas_en@cas.cn

