
They Are Hijacked: Sequestration of Cellular Interacting Proteins by Polyglutamine-expanded Ataxin-3
Oct 20, 2014 Email"> PrintText Size
Some neurodegenerative diseases or conformational diseases are caused by aberrant protein misfolding and aggregation. Polyglutamine (polyQ) expansion can make the disease-related proteins readily misfolded and form insoluble aggregates or inclusions. However, how the protein aggregates lead to cellular dysfunction and cytotoxicity remains elusive.
Recently, a research team led by Dr. HU Hongyu from the Institute of Biochemistry and Cell Biology (SIBCB), Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences revealed that aggregation of polyQ-expanded ataxin-3(Atx3) sequesters its specific interacting partners into inclusions and thus impairs the cellular function of these essential proteins.
YANG Hui and her colleagues in Dr. HU’s lab found that polyQ-expanded Atx3 sequesters P97/VCP and ubiquitin conjugates into the protein inclusions through specific interactions both in vitro and in cells. This specific sequestration impairs the normal cellular function of P97/VCP in down-regulating neddylation. However, expansion of the polyQ tract in Atx3 does not alter the conformation of its surrounding regions and the interaction affinities with its interacting partners, although it indeed facilitates misfolding and aggregation of the Atx3 protein. Thus, a loss-of-function pathology was provided that the direct effect of polyQ expansion is to entice the protein prone to aggregation, which, during aggregating process, alters its state from soluble to insoluble and simultaneously sequesters its interacting partners into the insolubilities.
Dr. HU’s lab previously reported that P97 down-regulates the neddylation in cells (Liu et al., J Biol Chem, 2013). Based on this study, the researchers revealed that overexpression of polyQ-expanded Atx3 significantly increases the neddylation level compared with that of wild-type Atx3. However, when overexpressed the mutant of polyQ-expanded Atx3 that cannot interact with P97, the neddylation level goes back to that of wild-type Atx3. This implies that specific interaction is critical to impairing the normal cellular function of P97 in down-regulating neddylation by polyQ-expanded Atx3.
Fujita et al. reported that it is the polyQ tract that directly mediates the interaction between polyQ-expanded proteins (such as huntingtin, Atx1 and Atx7) and P97, and consequently affects the function of P97 (Fujita et al., Nat Commun, 2013). However, the researchers argued that this effect can be defined as non-specific sequestration mediated by indirect protein association owning to involvement of P97 in quality control. They speculated that specific protein interaction is critical for the sequestration of P97 and other interacting partners by polyQ-expanded Atx3, which can be recognized as specific sequestration.
Further biophysical studies also elucidated that aggregation of the polyQ expansion in Atx3 does not affect the conformation of its surrounding regions and the interaction affinities with P97 or K48-linked diubiquitin. Thus, the direct effect of polyQ expansion is to entice the protein prone to aggregation and alter its state from soluble to insoluble, and simultaneously sequesters its interacting partners into the inclusions during aggregation process.
Based on these findings, the researchers proposed a loss-of-function pathology that polyQ aggregates cause cellular dysfunction and cytotoxicity by deteriorating the interacting partners in cell, which will be helpful for deciphering pathologies of the neurodegenerative diseases and discovering pharmaceutical therapies.
This work entitled “Aggregation of polyglutamine-expanded ataxin-3 sequesters its specific interacting partners into inclusions: Implication in a loss-of-function pathology” was published online in Scientific Reports on September 18, 2014.
The work was supported by the Ministry of Science and Technology, the National Natural Science Foundation of China, and the Chinese Academy of Sciences.
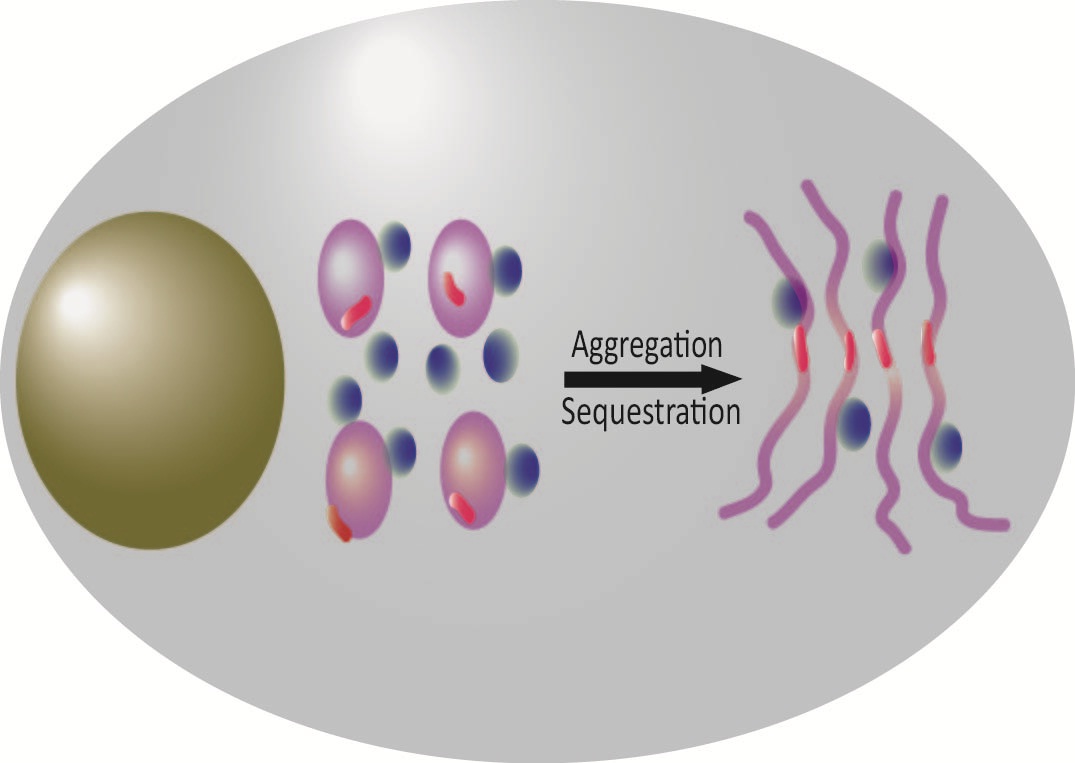
PolyQ aggregates to hijack the specific interacting partners: the polyQ protein self-aggregates and sequesters its cellular interacting proteins into insolubilities and this snowball-like sequestration effect may cause some related proteins dysfunctional and cytotoxic. (Image by Dr. HU Hongyu’s group)
Some neurodegenerative diseases or conformational diseases are caused by aberrant protein misfolding and aggregation. Polyglutamine (polyQ) expansion can make the disease-related proteins readily misfolded and form insoluble aggregates or inclusions. However, how the protein aggregates lead to cellular dysfunction and cytotoxicity remains elusive.
Recently, a research team led by Dr. HU Hongyu from the Institute of Biochemistry and Cell Biology (SIBCB), Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences revealed that aggregation of polyQ-expanded ataxin-3(Atx3) sequesters its specific interacting partners into inclusions and thus impairs the cellular function of these essential proteins.
YANG Hui and her colleagues in Dr. HU’s lab found that polyQ-expanded Atx3 sequesters P97/VCP and ubiquitin conjugates into the protein inclusions through specific interactions both in vitro and in cells. This specific sequestration impairs the normal cellular function of P97/VCP in down-regulating neddylation. However, expansion of the polyQ tract in Atx3 does not alter the conformation of its surrounding regions and the interaction affinities with its interacting partners, although it indeed facilitates misfolding and aggregation of the Atx3 protein. Thus, a loss-of-function pathology was provided that the direct effect of polyQ expansion is to entice the protein prone to aggregation, which, during aggregating process, alters its state from soluble to insoluble and simultaneously sequesters its interacting partners into the insolubilities.
Dr. HU’s lab previously reported that P97 down-regulates the neddylation in cells (Liu et al., J Biol Chem, 2013). Based on this study, the researchers revealed that overexpression of polyQ-expanded Atx3 significantly increases the neddylation level compared with that of wild-type Atx3. However, when overexpressed the mutant of polyQ-expanded Atx3 that cannot interact with P97, the neddylation level goes back to that of wild-type Atx3. This implies that specific interaction is critical to impairing the normal cellular function of P97 in down-regulating neddylation by polyQ-expanded Atx3.
Fujita et al. reported that it is the polyQ tract that directly mediates the interaction between polyQ-expanded proteins (such as huntingtin, Atx1 and Atx7) and P97, and consequently affects the function of P97 (Fujita et al., Nat Commun, 2013). However, the researchers argued that this effect can be defined as non-specific sequestration mediated by indirect protein association owning to involvement of P97 in quality control. They speculated that specific protein interaction is critical for the sequestration of P97 and other interacting partners by polyQ-expanded Atx3, which can be recognized as specific sequestration.
Further biophysical studies also elucidated that aggregation of the polyQ expansion in Atx3 does not affect the conformation of its surrounding regions and the interaction affinities with P97 or K48-linked diubiquitin. Thus, the direct effect of polyQ expansion is to entice the protein prone to aggregation and alter its state from soluble to insoluble, and simultaneously sequesters its interacting partners into the inclusions during aggregation process.
Based on these findings, the researchers proposed a loss-of-function pathology that polyQ aggregates cause cellular dysfunction and cytotoxicity by deteriorating the interacting partners in cell, which will be helpful for deciphering pathologies of the neurodegenerative diseases and discovering pharmaceutical therapies.
This work entitled “Aggregation of polyglutamine-expanded ataxin-3 sequesters its specific interacting partners into inclusions: Implication in a loss-of-function pathology” was published online in Scientific Reports on September 18, 2014.
The work was supported by the Ministry of Science and Technology, the National Natural Science Foundation of China, and the Chinese Academy of Sciences.

PolyQ aggregates to hijack the specific interacting partners: the polyQ protein self-aggregates and sequesters its cellular interacting proteins into insolubilities and this snowball-like sequestration effect may cause some related proteins dysfunctional and cytotoxic. (Image by Dr. HU Hongyu’s group)
CAS Institutes
There are 124 Institutions directly under the CAS by the end of 2012, with 104 research institutes, five universities & supporting organizations, 12 management organizations that consist of the headquarters and branches, and three other units. Moreover, there are 25 legal entities affiliated and 22 CAS invested holding enterprisesThere are 124 I...>> more
Contact Us

Chinese Academy of Sciences
Add: 52 Sanlihe Rd., Xicheng District, Beijing, China
Postcode: 100864
Tel: 86-10-68597592 (day) 86-10-68597289 (night)
Fax: 86-10-68511095 (day) 86-10-68512458 (night)
E-mail: cas_en@cas.cn

