Protein Translation Mechanism Brings Clue to Design New Antibiotics
Sep 22, 2014 Email"> PrintText Size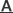
Chinese scientists have revealed a new mechanism during protein translation, a finding that helps to clarify the pathogenic mechanisms of many diseases closely related to human health and provides new strategies for the development of novel antibiotics and effective treatments. The Nature Structural & Molecular Biology magazine reported this breakthrough.Ribosomes serve as engineers in the chemical factories existing in all organisms. They translate the code carried in gene sequences, according to which different kinds of proteins can be produced to control chemical processes in the organisms. Since three scientists won the Nobel Prize in 2009 for their ribosomal studies, ribosome has become an even hotter research spot to the science community.In the process of translation, elongation factor G (EF-G) plays an important role in catalytic function, which pushes the ribosome moving along the mRNA from 5’ to 3’. But how EF-G initiates translocation remains a key unresolved question. A research team headed by Professor QIN Yan from the Institute of Biophysics (IBP) of Chinese Academy of Sciences recently provided critical insights to answer the question.In order to study the function of EF-G, the team used Escherichia coli as model organism and performed systematic mutagenesis and conducted structural and functional assays. They found that the interactions between the decoding center and the codon–anticodon duplex constitute the barrier for translocation. EF-G contains five domains, the domains of I, II, III and V associated with the binding to ribosome, and the domain IV is the key part of catalytic functions. In the process of mutant EF-G functional research, QIN’s team found that the mutations on the two loops have a great influence on the catalytic ability of EF-G. Biochemical experiment results showed that, in the process of translocation, EF-G domains I-III and V stabilize the ribosome in the relative rotation conformation, while domain IV reaches into ribosome decoding center, disturbs and eventually replaces codon-anticodon double helix interaction in the decoding center.Professor QIN’s team has studied ribosomal translocation mechanism for many years. This work is not only a major breakthrough in the study of EF-G, but also provides molecular basis to understand how ribosome function in life processes. The team also found for the first time that EF4 can move ribosome backwards on mRNA. This new description of ribosome movement is the greatest contribution of the research. Based on the EF-G and EF4 catalytic mechanism, researchers may intervene the prokaryotic translation process, thus design new antibiotics."We found that EF4 and non-coding RNA, mainly some long non-coding RNA, together regulate translation of proteins in tumor cells, thereby influencing tumour formation, degradation and transfer, which are essential for diagnosis, treatment of cancer patients." Professor QIN said. Their results revealed clear molecular mechanism of ribosomal function which helps to recognize pathogenic mechanisms of many diseases and to identify effective treatments.The article was entitled "EF-G catalyzes tRNA translocation by disrupting interactions between decoding center and codon–anticodonduplex". Figure: The Mechanism and Working Model of EF-G-catalyzed Translocation (Image by IBP)
Chinese scientists have revealed a new mechanism during protein translation, a finding that helps to clarify the pathogenic mechanisms of many diseases closely related to human health and provides new strategies for the development of novel antibiotics and effective treatments. The Nature Structural & Molecular Biology magazine reported this breakthrough.
Ribosomes serve as engineers in the chemical factories existing in all organisms. They translate the code carried in gene sequences, according to which different kinds of proteins can be produced to control chemical processes in the organisms. Since three scientists won the Nobel Prize in 2009 for their ribosomal studies, ribosome has become an even hotter research spot to the science community.
In the process of translation, elongation factor G (EF-G) plays an important role in catalytic function, which pushes the ribosome moving along the mRNA from 5’ to 3’. But how EF-G initiates translocation remains a key unresolved question. A research team headed by Professor QIN Yan from the Institute of Biophysics (IBP) of Chinese Academy of Sciences recently provided critical insights to answer the question.
In order to study the function of EF-G, the team used Escherichia coli as model organism and performed systematic mutagenesis and conducted structural and functional assays. They found that the interactions between the decoding center and the codon–anticodon duplex constitute the barrier for translocation. EF-G contains five domains, the domains of I, II, III and V associated with the binding to ribosome, and the domain IV is the key part of catalytic functions. In the process of mutant EF-G functional research, QIN’s team found that the mutations on the two loops have a great influence on the catalytic ability of EF-G. Biochemical experiment results showed that, in the process of translocation, EF-G domains I-III and V stabilize the ribosome in the relative rotation conformation, while domain IV reaches into ribosome decoding center, disturbs and eventually replaces codon-anticodon double helix interaction in the decoding center.
Professor QIN’s team has studied ribosomal translocation mechanism for many years. This work is not only a major breakthrough in the study of EF-G, but also provides molecular basis to understand how ribosome function in life processes. The team also found for the first time that EF4 can move ribosome backwards on mRNA. This new description of ribosome movement is the greatest contribution of the research. Based on the EF-G and EF4 catalytic mechanism, researchers may intervene the prokaryotic translation process, thus design new antibiotics.
"We found that EF4 and non-coding RNA, mainly some long non-coding RNA, together regulate translation of proteins in tumor cells, thereby influencing tumour formation, degradation and transfer, which are essential for diagnosis, treatment of cancer patients." Professor QIN said. Their results revealed clear molecular mechanism of ribosomal function which helps to recognize pathogenic mechanisms of many diseases and to identify effective treatments.
The article was entitled "EF-G catalyzes tRNA translocation by disrupting interactions between decoding center and codon–anticodonduplex".
Figure: The Mechanism and Working Model of EF-G-catalyzed Translocation (Image by IBP)
CAS Institutes
There are 124 Institutions directly under the CAS by the end of 2012, with 104 research institutes, five universities & supporting organizations, 12 management organizations that consist of the headquarters and branches, and three other units. Moreover, there are 25 legal entities affiliated and 22 CAS invested holding enterprisesThere are 124 I...>> more
Contact Us

Chinese Academy of Sciences
Add: 52 Sanlihe Rd., Xicheng District, Beijing, China
Postcode: 100864
Tel: 86-10-68597592 (day) 86-10-68597289 (night)
Fax: 86-10-68511095 (day) 86-10-68512458 (night)
E-mail: cas_en@cas.cn