
A Stable-and-Active Catalyst Tailored for CO2 Conversion
Jul 17, 2014 Email"> PrintText Size
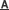
CO2 conversion into high-value products is of great strategic significance for utilization of this greenhouse gas. Specifically, the synthesis of cyclic carbonates is a very typical way for efficient transformation of CO2, which has long been a hot issue in catalytic field. However, for commonly-used heterogeneous catalysts, some disadvantages such as scarce active centers for inorganic catalysts and poor thermal stability for organic ones still remain to be overcome for long-term effective industrial application. Thus, it is essential to explore a new heterogeneous catalyst with more active centers and a higher stability.
From this point, the research group led by Prof. ZHANG Suojiang at Institute of Process Engineering (IPE), Chinese Academy of Sciences, developed a certain kind of graphitic carbon nitride (g-C3N4) as a heterogeneous catalyst for the synthesis of cyclic carbonates, making up the disadvantages of both inorganic and organic catalysts.
Recent reports showed that edge defects are key active sites in defect-containing g-C3N4 for CO2 activation. Enlightened by this, the research group selected urea as precursor for g-C3N4 preparation. The template-free method avoided damage caused by conventional template-removal process, and made more edge defects available. The urea-derived g-C3N4 exhibited higher catalytic activity and stability than the common bulk catalysts for cyclic carbonate synthesis. Furthermore, by adjusting preparation conditions of the catalyst for better performance, the group provides a new method for upgrading catalyst.
The results have been published on Catalysis Science & Technology. 2014, 4(6):1556-1562.
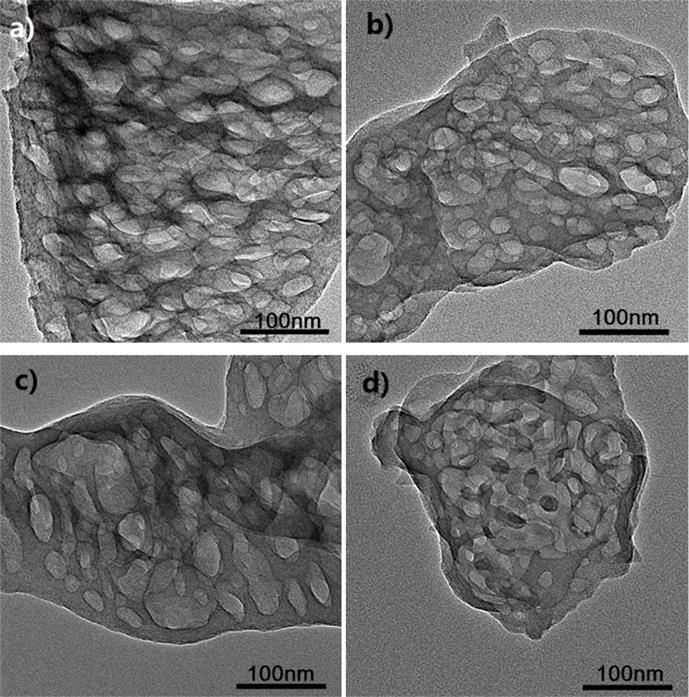
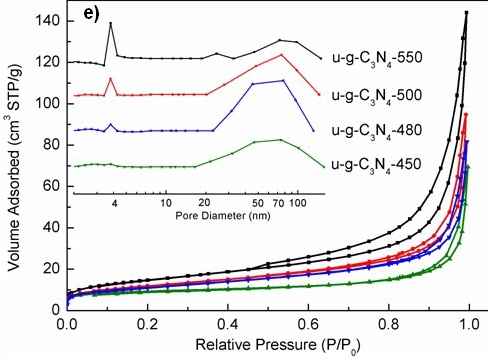
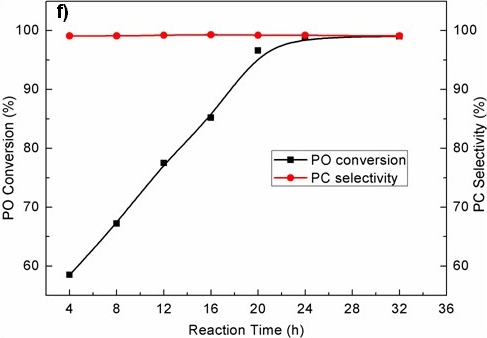
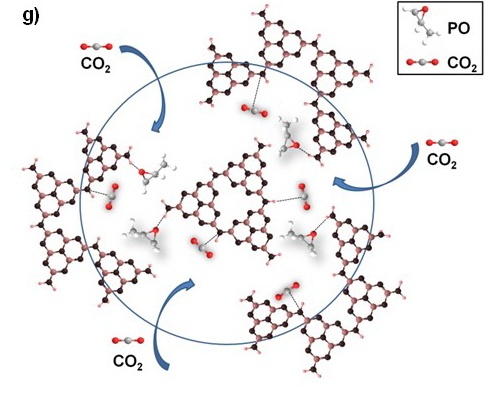
Figure TEM micrographs of graphitic carbon nitrides: a). u-g-C3N4-550, b). u-g-C3N4-500, c). u-g-C3N4-480, d). u-g-C3N4-450; e). N2 adsorption–desorption isotherms of prepared u-g-C3N4; f). The catalytic activity tests of u-g-C3N4-480; g). Illustration for understanding the u-g-C3N4 activation in the reaction. (Image by IPE)
CO2 conversion into high-value products is of great strategic significance for utilization of this greenhouse gas. Specifically, the synthesis of cyclic carbonates is a very typical way for efficient transformation of CO2, which has long been a hot issue in catalytic field. However, for commonly-used heterogeneous catalysts, some disadvantages such as scarce active centers for inorganic catalysts and poor thermal stability for organic ones still remain to be overcome for long-term effective industrial application. Thus, it is essential to explore a new heterogeneous catalyst with more active centers and a higher stability.
From this point, the research group led by Prof. ZHANG Suojiang at Institute of Process Engineering (IPE), Chinese Academy of Sciences, developed a certain kind of graphitic carbon nitride (g-C3N4) as a heterogeneous catalyst for the synthesis of cyclic carbonates, making up the disadvantages of both inorganic and organic catalysts.
Recent reports showed that edge defects are key active sites in defect-containing g-C3N4 for CO2 activation. Enlightened by this, the research group selected urea as precursor for g-C3N4 preparation. The template-free method avoided damage caused by conventional template-removal process, and made more edge defects available. The urea-derived g-C3N4 exhibited higher catalytic activity and stability than the common bulk catalysts for cyclic carbonate synthesis. Furthermore, by adjusting preparation conditions of the catalyst for better performance, the group provides a new method for upgrading catalyst.
The results have been published on Catalysis Science & Technology. 2014, 4(6):1556-1562.




Figure TEM micrographs of graphitic carbon nitrides: a). u-g-C3N4-550, b). u-g-C3N4-500, c). u-g-C3N4-480, d). u-g-C3N4-450; e). N2 adsorption–desorption isotherms of prepared u-g-C3N4; f). The catalytic activity tests of u-g-C3N4-480; g). Illustration for understanding the u-g-C3N4 activation in the reaction. (Image by IPE)
CAS Institutes
There are 124 Institutions directly under the CAS by the end of 2012, with 104 research institutes, five universities & supporting organizations, 12 management organizations that consist of the headquarters and branches, and three other units. Moreover, there are 25 legal entities affiliated and 22 CAS invested holding enterprisesThere are 124 I...>> more
Contact Us

Chinese Academy of Sciences
Add: 52 Sanlihe Rd., Xicheng District, Beijing, China
Postcode: 100864
Tel: 86-10-68597592 (day) 86-10-68597289 (night)
Fax: 86-10-68511095 (day) 86-10-68512458 (night)
E-mail: cas_en@cas.cn

