
Scientists Uncover IRE1α-XBP1 Pathway Regulates Compensatory Proliferation of Pancreatic β-cells
Jun 30, 2014 Email"> PrintText Size
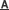
In eukaryotes, increased protein folding demand at the endoplasmic reticulum (ER) activates the unfolded protein response (UPR), which plays a pivotal role in control of cellular functions and survival under ER stress. Inositol-requiring enzyme 1 (IRE1), an ER-resident transmembrane Ser/Thr protein kinase and endoribonuclease, is the most conserved ER stress sensor that mediates a key branch of the UPR. In mammals, activation of IRE1α results in non-conventional splicing of the mRNA encoding the transcription factor X-box binding protein 1 (XBP1), generating a spliced active form of XBP1 (XBP1s) to initiate a major UPR program. The IRE1-XBP1 pathway has been implicated in the homeostatic regulation of pancreatic islet β-cells. However, the role in vivo of IRE1α in integrating metabolic ER stress signals to regulate β-cell functions remains poorly understood.
LIU Yong, professor of the Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, and group members demonstrated that IRE1α in pancreatic β-cells is not only involved in regulation of insulin biosynthesis, but also is linked to the cell cycle machinery in promoting the compensatory proliferation of β-cells in the face of obesity and insulin resistance. Their findings provided a better understanding of the connections between the ER stress signaling and the proliferative control of β-cells.
In the study, researchers developed conditional Ire1α knockout mice (Ire1αf/f:Cre) by crossisng floxed Ire1α mice the transgenic RIP-Cre mice.
They found that deletion of Ire1α specifically in pancreatic β cells results in hyperglycemia and glucose intolerance. When fed a normal chow diet, Ire1αf/f:Cre mice had reduced serum insulin levels and lower islet insulin content without causing obvious alterations in islet morphology. In contrast, when challenged with a high-fat diet (HFD), Ire1αf/f:Cre mice displayed significant reductions in the number and size of islets as well as decreased proliferation of β-cells.
Furthermore, this defect in obesity-induced pancreatic β-cell expansion is associated with dysregulated expression of Cyclin D1, a critical proliferation regulator that can be directly regulated by the IRE1α-XBP1 pathway.
The article, entitled “The IRE1α-XBP1 pathway regulates metabolic stress induced compensatory proliferation of pancreatic β-cells”, was published online in Cell Research in May. This study was funded by grants from the National Basic Research 973 Program, the National Natural Science Foundation of China, and the Chinese Academy of Sciences.
Contact:
LIU Yong, Ph.D., Principal Investigator
Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences,
Shanghai 200031, China.
Tel: 86-21-54920244;
Fax: 86-21-54920291;
E-mail: liuy@sibs.ac.cn
The IRE1α-XBP1 pathway regulates metabolic stress-induced compensatory proliferation. (Image by Dr. LIU Yong's research group)
In eukaryotes, increased protein folding demand at the endoplasmic reticulum (ER) activates the unfolded protein response (UPR), which plays a pivotal role in control of cellular functions and survival under ER stress. Inositol-requiring enzyme 1 (IRE1), an ER-resident transmembrane Ser/Thr protein kinase and endoribonuclease, is the most conserved ER stress sensor that mediates a key branch of the UPR. In mammals, activation of IRE1α results in non-conventional splicing of the mRNA encoding the transcription factor X-box binding protein 1 (XBP1), generating a spliced active form of XBP1 (XBP1s) to initiate a major UPR program. The IRE1-XBP1 pathway has been implicated in the homeostatic regulation of pancreatic islet β-cells. However, the role in vivo of IRE1α in integrating metabolic ER stress signals to regulate β-cell functions remains poorly understood.
LIU Yong, professor of the Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, and group members demonstrated that IRE1α in pancreatic β-cells is not only involved in regulation of insulin biosynthesis, but also is linked to the cell cycle machinery in promoting the compensatory proliferation of β-cells in the face of obesity and insulin resistance. Their findings provided a better understanding of the connections between the ER stress signaling and the proliferative control of β-cells.
In the study, researchers developed conditional Ire1α knockout mice (Ire1αf/f:Cre) by crossisng floxed Ire1α mice the transgenic RIP-Cre mice.
They found that deletion of Ire1α specifically in pancreatic β cells results in hyperglycemia and glucose intolerance. When fed a normal chow diet, Ire1αf/f:Cre mice had reduced serum insulin levels and lower islet insulin content without causing obvious alterations in islet morphology. In contrast, when challenged with a high-fat diet (HFD), Ire1αf/f:Cre mice displayed significant reductions in the number and size of islets as well as decreased proliferation of β-cells.
Furthermore, this defect in obesity-induced pancreatic β-cell expansion is associated with dysregulated expression of Cyclin D1, a critical proliferation regulator that can be directly regulated by the IRE1α-XBP1 pathway.
The article, entitled “The IRE1α-XBP1 pathway regulates metabolic stress induced compensatory proliferation of pancreatic β-cells”, was published online in Cell Research in May. This study was funded by grants from the National Basic Research 973 Program, the National Natural Science Foundation of China, and the Chinese Academy of Sciences.
Contact:
LIU Yong, Ph.D., Principal Investigator
Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences,
Shanghai 200031, China.
Tel: 86-21-54920244;
Fax: 86-21-54920291;
E-mail: liuy@sibs.ac.cn

The IRE1α-XBP1 pathway regulates metabolic stress-induced compensatory proliferation. (Image by Dr. LIU Yong's research group)
CAS Institutes
There are 124 Institutions directly under the CAS by the end of 2012, with 104 research institutes, five universities & supporting organizations, 12 management organizations that consist of the headquarters and branches, and three other units. Moreover, there are 25 legal entities affiliated and 22 CAS invested holding enterprisesThere are 124 I...>> more
Contact Us

Chinese Academy of Sciences
Add: 52 Sanlihe Rd., Xicheng District, Beijing, China
Postcode: 100864
Tel: 86-10-68597592 (day) 86-10-68597289 (night)
Fax: 86-10-68511095 (day) 86-10-68512458 (night)
E-mail: cas_en@cas.cn

