
Novel Second Substituted Reaction of Oxo Clusters Reported
Jun 03, 2014 Email"> PrintText Size
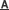
Compared with lacunary oxo-tungsten clusters, the lacunary oxo-vanadium (V-O) clusters are very unstable and difficult to be isolated, resulting in that no available lacunary V-O clusters can be used as the precursors. Generally, their substitution reactions are in-situ realized. In V-O clusters, the Keggin-type V18O42 unit is common, which has eighteen V=O groups on the cluster cage. Some V=O groups on the V18O42 cage can be replaced in the forming process of clusters. For example, the AsIII2O units replace the V=O groups to form the first substitution products formularized as As2nV18-nO42 (n = 2, 3, 4 …).
Then, the similar Sb2nV18-nO42 clusters were also obtained by replacing V=O groups with SbIII2O units. Moreover, the first substitution products with formula of Ge2nV18-nO42+2n (n = 2, 3, 4 …) have been successfully made by the substitution of Ge2O3 units. The introduction of the substituted atoms with different attribute expands the research field of the oxo-cluster chemistry and introduces the new properties for the V-O clusters, as well as will further tune their structures and properties.
Recently, Prof. YANG Guoyu’s group at Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, has made great progress on the second substitution reaction of the oxo clusters, and obtained two unprecedented V-O cluster open frameworks constructed from tetra-CdII-substituted Cd4Ge8V10O46 {Cd4Ge8V10} cluster units and GeO4 bridging groups under hydrothermal conditions, in which the {Cd4Ge8V10} clusters are formed via the first substitution reaction of the main-group oxo metal groups (Ge2O3) and then the second substitution reaction of the late transition metal Cd(II) cations, respectively.
It is the first 3-D vanadogermanates (VGOs) frameworks built by the largest number of TM-substituted {Cd4Ge8V10} cluster shells linked by inorganic tetrahedral GeO4 units and {V4} clusters, of which the {Cd4Ge8V10} cluster contains three different kinds metal cations. Notice that the second substitution products conforms the formula MmGe2nV18-n-mO42+2n-m (M = transition metal, n = 2, 3, 4 …; m = 2, 4…).
The result has been published in J. Am. Chem. Soc. (2014, 136, 5065), which not only exploits novel second substitution reaction on the V18O42 cluster cage, but also establishes the basis of the functionality on the V18O42 cluster, owing to introducing the different metal cations. Therefore, it possesses important guidance significance for the synthesis and functionality of the novel substituted oxo clusters, which will open up a frontier field of polyoxometalate chemistry.
Previously, Prof. YANG's group had also made a series of relevant progress on the V-O clusters. For instance, the Zn(II), Ni(II), and Cd(II) cations had been introduced into As2nV18-nO42 cluster skeleton and replaced some V=O groups on cluster cages to build up the novel second substitution products with the formula: MmAs2nV18-n-mO42-m (n = 2, 3, 4… m = 1, 2 …), which have been published in Eur. J. Inorg. Chem. (2004, 2004) and Inorg. Chem. (2004, 43, 8005; 2005, 44, 2426; 2007, 46, 9503), respectively. Moreover, the second substitution reaction was successfully extended to the Ge2nV18-nO42+2n system and made di-CdII-substituted VGO (Chem. Eur. J., 2010, 16, 13253).

The 3-D framework built by GeO4 groups, {Cd4Ge8V10} and {V4} clusters, showing the ferrimagnetic property (Image by Prof. YANG's Group)
Compared with lacunary oxo-tungsten clusters, the lacunary oxo-vanadium (V-O) clusters are very unstable and difficult to be isolated, resulting in that no available lacunary V-O clusters can be used as the precursors. Generally, their substitution reactions are in-situ realized. In V-O clusters, the Keggin-type V18O42 unit is common, which has eighteen V=O groups on the cluster cage. Some V=O groups on the V18O42 cage can be replaced in the forming process of clusters. For example, the AsIII2O units replace the V=O groups to form the first substitution products formularized as As2nV18-nO42 (n = 2, 3, 4 …).
Then, the similar Sb2nV18-nO42 clusters were also obtained by replacing V=O groups with SbIII2O units. Moreover, the first substitution products with formula of Ge2nV18-nO42+2n (n = 2, 3, 4 …) have been successfully made by the substitution of Ge2O3 units. The introduction of the substituted atoms with different attribute expands the research field of the oxo-cluster chemistry and introduces the new properties for the V-O clusters, as well as will further tune their structures and properties.
Recently, Prof. YANG Guoyu’s group at Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, has made great progress on the second substitution reaction of the oxo clusters, and obtained two unprecedented V-O cluster open frameworks constructed from tetra-CdII-substituted Cd4Ge8V10O46 {Cd4Ge8V10} cluster units and GeO4 bridging groups under hydrothermal conditions, in which the {Cd4Ge8V10} clusters are formed via the first substitution reaction of the main-group oxo metal groups (Ge2O3) and then the second substitution reaction of the late transition metal Cd(II) cations, respectively.
It is the first 3-D vanadogermanates (VGOs) frameworks built by the largest number of TM-substituted {Cd4Ge8V10} cluster shells linked by inorganic tetrahedral GeO4 units and {V4} clusters, of which the {Cd4Ge8V10} cluster contains three different kinds metal cations. Notice that the second substitution products conforms the formula MmGe2nV18-n-mO42+2n-m (M = transition metal, n = 2, 3, 4 …; m = 2, 4…).
The result has been published in J. Am. Chem. Soc. (2014, 136, 5065), which not only exploits novel second substitution reaction on the V18O42 cluster cage, but also establishes the basis of the functionality on the V18O42 cluster, owing to introducing the different metal cations. Therefore, it possesses important guidance significance for the synthesis and functionality of the novel substituted oxo clusters, which will open up a frontier field of polyoxometalate chemistry.
Previously, Prof. YANG's group had also made a series of relevant progress on the V-O clusters. For instance, the Zn(II), Ni(II), and Cd(II) cations had been introduced into As2nV18-nO42 cluster skeleton and replaced some V=O groups on cluster cages to build up the novel second substitution products with the formula: MmAs2nV18-n-mO42-m (n = 2, 3, 4… m = 1, 2 …), which have been published in Eur. J. Inorg. Chem. (2004, 2004) and Inorg. Chem. (2004, 43, 8005; 2005, 44, 2426; 2007, 46, 9503), respectively. Moreover, the second substitution reaction was successfully extended to the Ge2nV18-nO42+2n system and made di-CdII-substituted VGO (Chem. Eur. J., 2010, 16, 13253).


The 3-D framework built by GeO4 groups, {Cd4Ge8V10} and {V4} clusters, showing the ferrimagnetic property (Image by Prof. YANG's Group)
CAS Institutes
There are 124 Institutions directly under the CAS by the end of 2012, with 104 research institutes, five universities & supporting organizations, 12 management organizations that consist of the headquarters and branches, and three other units. Moreover, there are 25 legal entities affiliated and 22 CAS invested holding enterprisesThere are 124 I...>> more
Contact Us

Chinese Academy of Sciences
Add: 52 Sanlihe Rd., Xicheng District, Beijing, China
Postcode: 100864
Tel: 86-10-68597592 (day) 86-10-68597289 (night)
Fax: 86-10-68511095 (day) 86-10-68512458 (night)
E-mail: cas_en@cas.cn

