
Researchers Reveal Structural Basis for Integrin Inactive State
May 30, 2014 Email"> PrintText Size
The immune surveillance and host defense depend on the trafficking of lymphocytes from the blood circulation to different tissues. Lymphocytes homing to the gut is mainly mediated by a cell adhesion molecule named integrin a4b7. The dynamic regulation of a4b7 affinity to the ligand, MAdCAM-1, allows this integrin to mediate both rolling and firm adhesion of lymphocyte, two critical steps during lymphocyte homing. Notably, most integrins are not constitutively activated, but exist in an inactive state in physiological conditions, which is very important for their normal biological functions. The fact that integrin activation is associated with displacements of the a1 and a7 helices in bI domain suggests the important roles of a1 and a7 helices in keeping integrin in low-affinity state, whereas the exquisite mechanism remains elusive.
In collaboration with Professor LI Guohui from Dalian Institute of Chemical Physics, Dr. LIU Jie and their colleagues led by Professor CHEN Jianfeng from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, discovered that the hydrophobic contacts between the center of bI domain and a1/a7 helices are crucial for the low-affinity state of integrin a4b7.
Using molecular dynamic simulation, they identified nine hydrophobic residues involved in the critical hydrophobic contacts for keeping integrin in low-affinity state. a4b7 not only showed high-affinity binding to soluble MAdCAM-1, but also supported firm cell adhesion to immobilized MAdCAM-1 in shear flow after disrupting these hydrophobic contacts. Disruption of the hydrophobic contacts also induced the active conformation of a4b7. Their findings reveal an important structural basis for integrin low-affinity state.
This work entitled “The hydrophobic contacts between the center of bI domain and a1/a7 helices are crucial for the low-affinity state of integrin a4b7” was published online in the FEBS Journal on May 7, 2014. It is funded by grants from the Ministry of Science and Technology of China, the National Natural Science Foundation of China, Chinese Academy of Sciences, and the Science and Technology Commission of Shanghai Municipality.
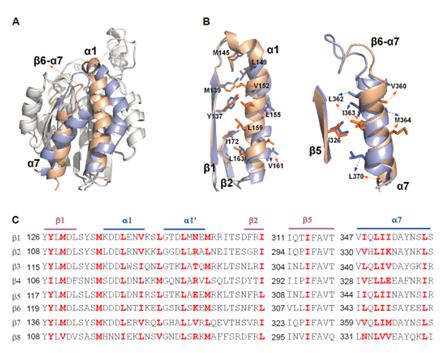
Superimposition of the closed (lightblue) and open (wheat) structures of b7I domain and sequence alignment of integrin b subunit I domain. Residues might involved in hydrophobic contacts formed by the a1 helix and a7 helix with their opposite hydrophobic faces are shown in stick in structure and red in sequence alignment. (Image by Prof. CHEN Jianfeng`s group)
The immune surveillance and host defense depend on the trafficking of lymphocytes from the blood circulation to different tissues. Lymphocytes homing to the gut is mainly mediated by a cell adhesion molecule named integrin a4b7. The dynamic regulation of a4b7 affinity to the ligand, MAdCAM-1, allows this integrin to mediate both rolling and firm adhesion of lymphocyte, two critical steps during lymphocyte homing. Notably, most integrins are not constitutively activated, but exist in an inactive state in physiological conditions, which is very important for their normal biological functions. The fact that integrin activation is associated with displacements of the a1 and a7 helices in bI domain suggests the important roles of a1 and a7 helices in keeping integrin in low-affinity state, whereas the exquisite mechanism remains elusive.
In collaboration with Professor LI Guohui from Dalian Institute of Chemical Physics, Dr. LIU Jie and their colleagues led by Professor CHEN Jianfeng from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, discovered that the hydrophobic contacts between the center of bI domain and a1/a7 helices are crucial for the low-affinity state of integrin a4b7.
Using molecular dynamic simulation, they identified nine hydrophobic residues involved in the critical hydrophobic contacts for keeping integrin in low-affinity state. a4b7 not only showed high-affinity binding to soluble MAdCAM-1, but also supported firm cell adhesion to immobilized MAdCAM-1 in shear flow after disrupting these hydrophobic contacts. Disruption of the hydrophobic contacts also induced the active conformation of a4b7. Their findings reveal an important structural basis for integrin low-affinity state.
This work entitled “The hydrophobic contacts between the center of bI domain and a1/a7 helices are crucial for the low-affinity state of integrin a4b7” was published online in the FEBS Journal on May 7, 2014. It is funded by grants from the Ministry of Science and Technology of China, the National Natural Science Foundation of China, Chinese Academy of Sciences, and the Science and Technology Commission of Shanghai Municipality.

Superimposition of the closed (lightblue) and open (wheat) structures of b7I domain and sequence alignment of integrin b subunit I domain. Residues might involved in hydrophobic contacts formed by the a1 helix and a7 helix with their opposite hydrophobic faces are shown in stick in structure and red in sequence alignment. (Image by Prof. CHEN Jianfeng`s group)
CAS Institutes
There are 124 Institutions directly under the CAS by the end of 2012, with 104 research institutes, five universities & supporting organizations, 12 management organizations that consist of the headquarters and branches, and three other units. Moreover, there are 25 legal entities affiliated and 22 CAS invested holding enterprisesThere are 124 I...>> more
Contact Us

Chinese Academy of Sciences
Add: 52 Sanlihe Rd., Xicheng District, Beijing, China
Postcode: 100864
Tel: 86-10-68597592 (day) 86-10-68597289 (night)
Fax: 86-10-68511095 (day) 86-10-68512458 (night)
E-mail: cas_en@cas.cn

