
Study Reveals Conserved Mechanism of Protein-RNA Recognition in Telomerase
May 26, 2014 Email"> PrintText Size
Telomeres, the ends of linear eukaryotic chromosomes, are highly specialized structures that are essential for genome integrity and stability. Telomerase, which is a large ribonucleoprotein complex minimally composed of a catalytic telomerase reverse transcriptase (TERT) and an RNA component (TR), is crucial for telomere maintenance and genome integrity. Mutations that disrupt telomerase function have been linked to numerous human diseases that arise from telomere shortening and genome instability. Despite the strong medical applications, the mechanism for telomerase holoenzyme assembly remains poorly understood.
HUANG Jing and her colleagues from a research group led by Prof. LEI Ming at National Center for Protein Science Shanghai, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, determined the crystal structure of conserved regions 4 and 5 (CR4/5), a conserved domain of vertebrate TR, complexed with the TR-binding domain (TRBD) of TERT.
This study is the first time providing an atomic level description of the protein-RNA interaction in vertebrate telomerase and revealing residues and structures crucial for telomerase ribonucleoprotein assembly.
In this protein-RNA heterodimeric structure, CR4/5 adopts an L-shaped three-way junction conformation with its two arms clamping onto TRBD. Both sequence and the conformation of CR4/5 are required for the interaction. The structural and mutational analyses strongly suggests that the observed CR4/5-TRBD interaction is common to species from yeast to humans, and CR4/5 in vertebrate TR might play a similar role in telomerase regulation as stem-loop IV in Tetrahymena TR. Telomerase is strongly upregulated in most cancer cells and has been studied as a plausible anti-cancer target. The high-resolution map of telomerase RNA-protein binding interface would offer new opportunities for the development of therapeutics that modulate telomerase activity for the treatment of human diseases.
This study entitled “Structural basis for protein-RNA recognition in telomerase” was published online in Nature Structural and molecular biology on May 4, 2014. This work was done in collaboration with Prof. CHEN Julian from Arizona State University, and supported by grants from the Ministry of Science and Technology of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, and the US National Institutes of Health.
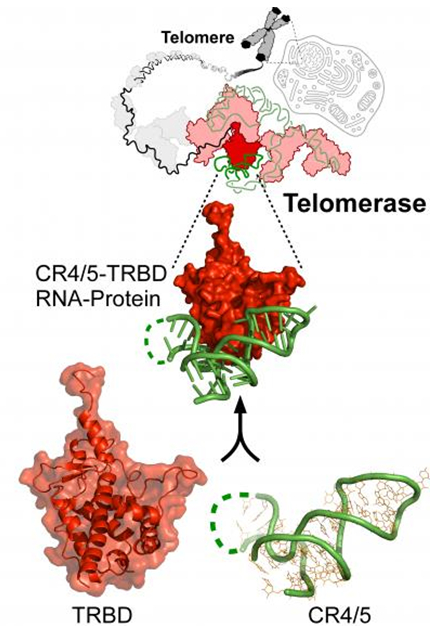
Model for the protein-RNA recognition in telomerase. (Image by Prof. LEI Ming’s group)
Telomeres, the ends of linear eukaryotic chromosomes, are highly specialized structures that are essential for genome integrity and stability. Telomerase, which is a large ribonucleoprotein complex minimally composed of a catalytic telomerase reverse transcriptase (TERT) and an RNA component (TR), is crucial for telomere maintenance and genome integrity. Mutations that disrupt telomerase function have been linked to numerous human diseases that arise from telomere shortening and genome instability. Despite the strong medical applications, the mechanism for telomerase holoenzyme assembly remains poorly understood.
HUANG Jing and her colleagues from a research group led by Prof. LEI Ming at National Center for Protein Science Shanghai, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, determined the crystal structure of conserved regions 4 and 5 (CR4/5), a conserved domain of vertebrate TR, complexed with the TR-binding domain (TRBD) of TERT.
This study is the first time providing an atomic level description of the protein-RNA interaction in vertebrate telomerase and revealing residues and structures crucial for telomerase ribonucleoprotein assembly.
In this protein-RNA heterodimeric structure, CR4/5 adopts an L-shaped three-way junction conformation with its two arms clamping onto TRBD. Both sequence and the conformation of CR4/5 are required for the interaction. The structural and mutational analyses strongly suggests that the observed CR4/5-TRBD interaction is common to species from yeast to humans, and CR4/5 in vertebrate TR might play a similar role in telomerase regulation as stem-loop IV in Tetrahymena TR. Telomerase is strongly upregulated in most cancer cells and has been studied as a plausible anti-cancer target. The high-resolution map of telomerase RNA-protein binding interface would offer new opportunities for the development of therapeutics that modulate telomerase activity for the treatment of human diseases.
This study entitled “Structural basis for protein-RNA recognition in telomerase” was published online in Nature Structural and molecular biology on May 4, 2014. This work was done in collaboration with Prof. CHEN Julian from Arizona State University, and supported by grants from the Ministry of Science and Technology of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, and the US National Institutes of Health.

Model for the protein-RNA recognition in telomerase. (Image by Prof. LEI Ming’s group)
CAS Institutes
There are 124 Institutions directly under the CAS by the end of 2012, with 104 research institutes, five universities & supporting organizations, 12 management organizations that consist of the headquarters and branches, and three other units. Moreover, there are 25 legal entities affiliated and 22 CAS invested holding enterprisesThere are 124 I...>> more
Contact Us

Chinese Academy of Sciences
Add: 52 Sanlihe Rd., Xicheng District, Beijing, China
Postcode: 100864
Tel: 86-10-68597592 (day) 86-10-68597289 (night)
Fax: 86-10-68511095 (day) 86-10-68512458 (night)
E-mail: cas_en@cas.cn

