
Scientists Reveal Molecular Mechanism of Smurf1 in Regulating Canonical Wnt Signaling Pathway
Apr 27, 2014 Email"> PrintText Size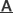
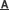
The Wnt/β-catenin signaling pathway is crucial in embryogenesis and its deregulation is linked to tumorigenesis and many human diseases. Recently, the regulations of Wnt/β-catenin signaling, including ubiquitin-mediated ones, have been intensively studied. Now, researchers from Chinese Academy of Sciences (CAS) reveal that the E3 ligase Smurf1 negatively regulates Wnt/β-catenin signaling, and that process requires its C2 domain and is cell-cycle dependent.
Under the supervision of Prof. LI Lin at the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Dr. FEI Cong and her colleagues have discovered a role of Smurf1 in regulating the Wnt/b-catenin signaling by ubiquitinating Axin through K29-linked poly-ubiquitination. Unexpectedly, Smurf1-mediated Axin ubiquitination does not lead to Axin degradation, but interrupts Axin-LRP5/6 association and then inhibits Wnt/β-catenin signaling. The atypical ubiquitination type and non-degradation fate of Axin discriminate Axin from other substrates of Smurf1.
Subsequent study further demonstrates that unlike most other substrates of Smurf1, Axin ubiquitination requires the C2 domain of Smurf1. On one hand, Smurf1’s C2 domain recruits Smurf1 to the plasma membrane for accessing Axin. On the other hand, Smurf1’s C2 domain, but not its WW domains, is directly involved in binding Axin, indicating a new example of WW-PY-independent Smurf1-substrate interaction which is mediated by Smurf1’s C2 domain. Finally, they showed that Smurf1-mediated Axin ubiquitination and its negative effect on Wnt/b-catenin signaling are attenuated in the G2/M phase of cell cycle, which contributes to an increase in the response of cells to Wnt stimulation.
The present paper entitled “Smurf1-mediated Axin ubiquitination requires Smurf1 C2 domain and is cell-cycle dependent” was published online in Journal of Biological Chemistry on April 3, 2014. Their previous paper entitled “Smurf1-mediated Lys29-linked Non-proteolytic Poly-ubiquitination of Axin Negatively Regulates Wnt/β-catenin Signaling” was published in Molecular and Cellular Biology on October 33, 2013.
The work was supported by grants from the Ministry of Science and Technology of China, and the National Natural Science Foundation of China.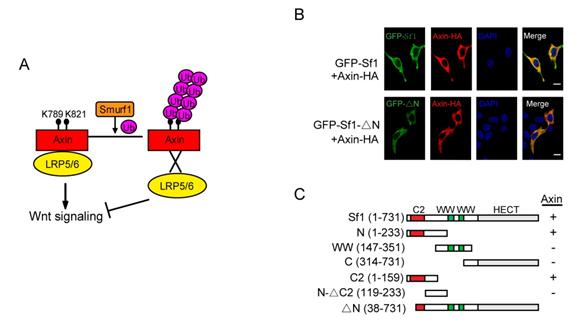
A. A proposed model for the role of Smurf1-mediated Axin ubiquitination in Wnt/b-catenin signaling: Smurf1 ubiquitinates Axin at the sites of K789 and K821 mainly through K29 ubiquitin linkage, and such modification does not trigger the degradation of Axin, instead, it disrupts Axin-LRP5/6 interaction and hence inhibits Wnt signaling transduction. B. Disruption of Smurf1 C2 domain abolished its co-localization with Axin aroung the cell membrane. C. Smurf1 C2 domain directly mediates its interaction with Axin. (Image by Prof. LI Lin’s group)
The Wnt/β-catenin signaling pathway is crucial in embryogenesis and its deregulation is linked to tumorigenesis and many human diseases. Recently, the regulations of Wnt/β-catenin signaling, including ubiquitin-mediated ones, have been intensively studied. Now, researchers from Chinese Academy of Sciences (CAS) reveal that the E3 ligase Smurf1 negatively regulates Wnt/β-catenin signaling, and that process requires its C2 domain and is cell-cycle dependent.
Under the supervision of Prof. LI Lin at the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Dr. FEI Cong and her colleagues have discovered a role of Smurf1 in regulating the Wnt/b-catenin signaling by ubiquitinating Axin through K29-linked poly-ubiquitination. Unexpectedly, Smurf1-mediated Axin ubiquitination does not lead to Axin degradation, but interrupts Axin-LRP5/6 association and then inhibits Wnt/β-catenin signaling. The atypical ubiquitination type and non-degradation fate of Axin discriminate Axin from other substrates of Smurf1.
Subsequent study further demonstrates that unlike most other substrates of Smurf1, Axin ubiquitination requires the C2 domain of Smurf1. On one hand, Smurf1’s C2 domain recruits Smurf1 to the plasma membrane for accessing Axin. On the other hand, Smurf1’s C2 domain, but not its WW domains, is directly involved in binding Axin, indicating a new example of WW-PY-independent Smurf1-substrate interaction which is mediated by Smurf1’s C2 domain. Finally, they showed that Smurf1-mediated Axin ubiquitination and its negative effect on Wnt/b-catenin signaling are attenuated in the G2/M phase of cell cycle, which contributes to an increase in the response of cells to Wnt stimulation.
The present paper entitled “Smurf1-mediated Axin ubiquitination requires Smurf1 C2 domain and is cell-cycle dependent” was published online in Journal of Biological Chemistry on April 3, 2014. Their previous paper entitled “Smurf1-mediated Lys29-linked Non-proteolytic Poly-ubiquitination of Axin Negatively Regulates Wnt/β-catenin Signaling” was published in Molecular and Cellular Biology on October 33, 2013.

A. A proposed model for the role of Smurf1-mediated Axin ubiquitination in Wnt/b-catenin signaling: Smurf1 ubiquitinates Axin at the sites of K789 and K821 mainly through K29 ubiquitin linkage, and such modification does not trigger the degradation of Axin, instead, it disrupts Axin-LRP5/6 interaction and hence inhibits Wnt signaling transduction. B. Disruption of Smurf1 C2 domain abolished its co-localization with Axin aroung the cell membrane. C. Smurf1 C2 domain directly mediates its interaction with Axin. (Image by Prof. LI Lin’s group)
CAS Institutes
There are 124 Institutions directly under the CAS by the end of 2012, with 104 research institutes, five universities & supporting organizations, 12 management organizations that consist of the headquarters and branches, and three other units. Moreover, there are 25 legal entities affiliated and 22 CAS invested holding enterprisesThere are 124 I...>> more
Contact Us

Chinese Academy of Sciences
Add: 52 Sanlihe Rd., Xicheng District, Beijing, China
Postcode: 100864
Tel: 86-10-68597592 (day) 86-10-68597289 (night)
Fax: 86-10-68511095 (day) 86-10-68512458 (night)
E-mail: cas_en@cas.cn

